
PDF Publication Title:
Text from PDF Page: 004
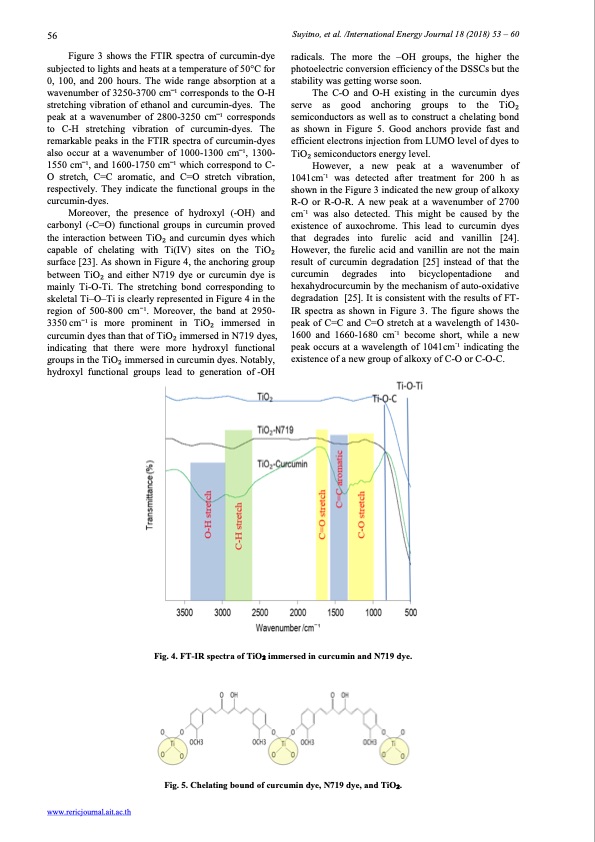
56 Suyitno, et al. /International Energy Journal 18 (2018) 53 – 60 Figure 3 shows the FTIR spectra of curcumin-dye subjected to lights and heats at a temperature of 50°C for 0, 100, and 200 hours. The wide range absorption at a wavenumber of 3250-3700 cm ̅1 corresponds to the O-H stretching vibration of ethanol and curcumin-dyes. The peak at a wavenumber of 2800-3250 cm ̅1 corresponds to C-H stretching vibration of curcumin-dyes. The remarkable peaks in the FTIR spectra of curcumin-dyes also occur at a wavenumber of 1000-1300 cm ̅1, 1300- 1550 cm ̅1, and 1600-1750 cm ̅1 which correspond to C- O stretch, C=C aromatic, and C=O stretch vibration, respectively. They indicate the functional groups in the curcumin-dyes. Moreover, the presence of hydroxyl (-OH) and carbonyl (-C=O) functional groups in curcumin proved the interaction between TiO2 and curcumin dyes which capable of chelating with Ti(IV) sites on the TiO2 surface [23]. As shown in Figure 4, the anchoring group between TiO2 and either N719 dye or curcumin dye is mainly Ti-O-Ti. The stretching bond corresponding to skeletal Ti–O–Ti is clearly represented in Figure 4 in the region of 500-800 cm−1. Moreover, the band at 2950- 3350 cm−1is more prominent in TiO2 immersed in curcumin dyes than that of TiO2 immersed in N719 dyes, indicating that there were more hydroxyl functional groups in the TiO2 immersed in curcumin dyes. Notably, hydroxyl functional groups lead to generation of -OH radicals. The more the –OH groups, the higher the photoelectric conversion efficiency of the DSSCs but the stability was getting worse soon. The C-O and O-H existing in the curcumin dyes serve as good anchoring groups to the TiO2 semiconductors as well as to construct a chelating bond as shown in Figure 5. Good anchors provide fast and efficient electrons injection from LUMO level of dyes to TiO2 semiconductors energy level. However, a new peak at a wavenumber of 1041cm-1 was detected after treatment for 200 h as shown in the Figure 3 indicated the new group of alkoxy R-O or R-O-R. A new peak at a wavenumber of 2700 cm-1 was also detected. This might be caused by the existence of auxochrome. This lead to curcumin dyes that degrades into furelic acid and vanillin [24]. However, the furelic acid and vanillin are not the main result of curcumin degradation [25] instead of that the curcumin degrades into bicyclopentadione and hexahydrocurcumin by the mechanism of auto-oxidative degradation [25]. It is consistent with the results of FT- IR spectra as shown in Figure 3. The figure shows the peak of C=C and C=O stretch at a wavelength of 1430- 1600 and 1660-1680 cm-1 become short, while a new - peak occurs at a wavelength of 1041cm 1 indicating the existence of a new group of alkoxy of C-O or C-O-C. www.rericjournal.ait.ac.th Fig. 4. FT-IR spectra of TiO2 immersed in curcumin and N719 dye. Fig. 5. Chelating bound of curcumin dye, N719 dye, and TiO2.PDF Image | Curcumin-Dye-Sensitized Solar Cells
PDF Search Title:
Curcumin-Dye-Sensitized Solar CellsOriginal File Name Searched:
10988664.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |