
PDF Publication Title:
Text from PDF Page: 004
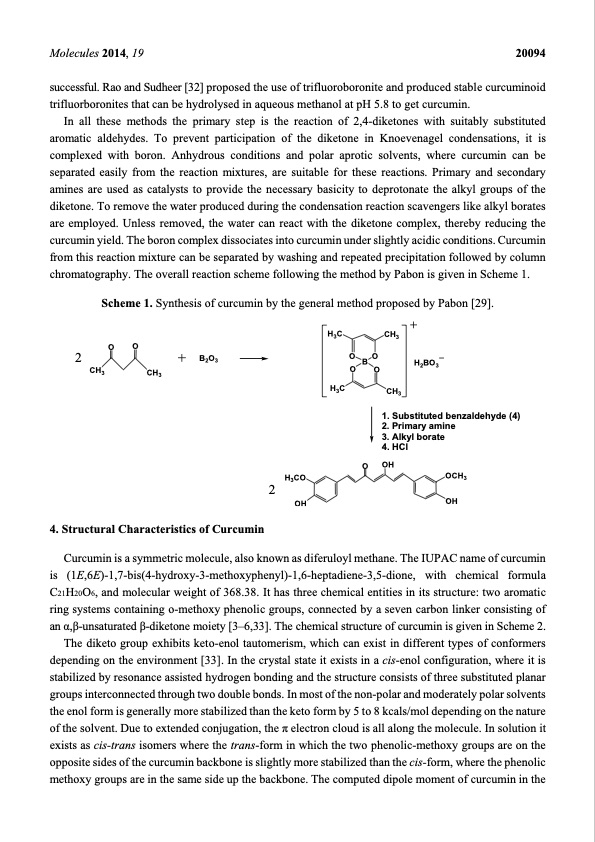
Molecules 2014, 19 20094 successful. Rao and Sudheer [32] proposed the use of trifluoroboronite and produced stable curcuminoid trifluorboronites that can be hydrolysed in aqueous methanol at pH 5.8 to get curcumin. In all these methods the primary step is the reaction of 2,4-diketones with suitably substituted aromatic aldehydes. To prevent participation of the diketone in Knoevenagel condensations, it is complexed with boron. Anhydrous conditions and polar aprotic solvents, where curcumin can be separated easily from the reaction mixtures, are suitable for these reactions. Primary and secondary amines are used as catalysts to provide the necessary basicity to deprotonate the alkyl groups of the diketone. To remove the water produced during the condensation reaction scavengers like alkyl borates are employed. Unless removed, the water can react with the diketone complex, thereby reducing the curcumin yield. The boron complex dissociates into curcumin under slightly acidic conditions. Curcumin from this reaction mixture can be separated by washing and repeated precipitation followed by column chromatography. The overall reaction scheme following the method by Pabon is given in Scheme 1. Scheme 1. Synthesis of curcumin by the general method proposed by Pabon [29]. 2 CH3 OO H3C H3C CH3 OBO H2BO3 OO B2O3 CH3 CH3 1. Substituted benzaldehyde (4) 2. Primary amine 3. Alkyl borate 4. HCl OH OCH3 OH Curcumin is a symmetric molecule, also known as diferuloyl methane. The IUPAC name of curcumin is (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, with chemical formula C21H20O6, and molecular weight of 368.38. It has three chemical entities in its structure: two aromatic ring systems containing o-methoxy phenolic groups, connected by a seven carbon linker consisting of an α,β-unsaturated β-diketone moiety [3–6,33]. The chemical structure of curcumin is given in Scheme 2. The diketo group exhibits keto-enol tautomerism, which can exist in different types of conformers depending on the environment [33]. In the crystal state it exists in a cis-enol configuration, where it is stabilized by resonance assisted hydrogen bonding and the structure consists of three substituted planar groups interconnected through two double bonds. In most of the non-polar and moderately polar solvents the enol form is generally more stabilized than the keto form by 5 to 8 kcals/mol depending on the nature of the solvent. Due to extended conjugation, the π electron cloud is all along the molecule. In solution it exists as cis-trans isomers where the trans-form in which the two phenolic-methoxy groups are on the opposite sides of the curcumin backbone is slightly more stabilized than the cis-form, where the phenolic methoxy groups are in the same side up the backbone. The computed dipole moment of curcumin in the O 4. Structural Characteristics of Curcumin 2 H3CO OHPDF Image | Curcumin: From Extraction to Therapeutic Agent
PDF Search Title:
Curcumin: From Extraction to Therapeutic AgentOriginal File Name Searched:
molecules-19-20091.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |