
PDF Publication Title:
Text from PDF Page: 003
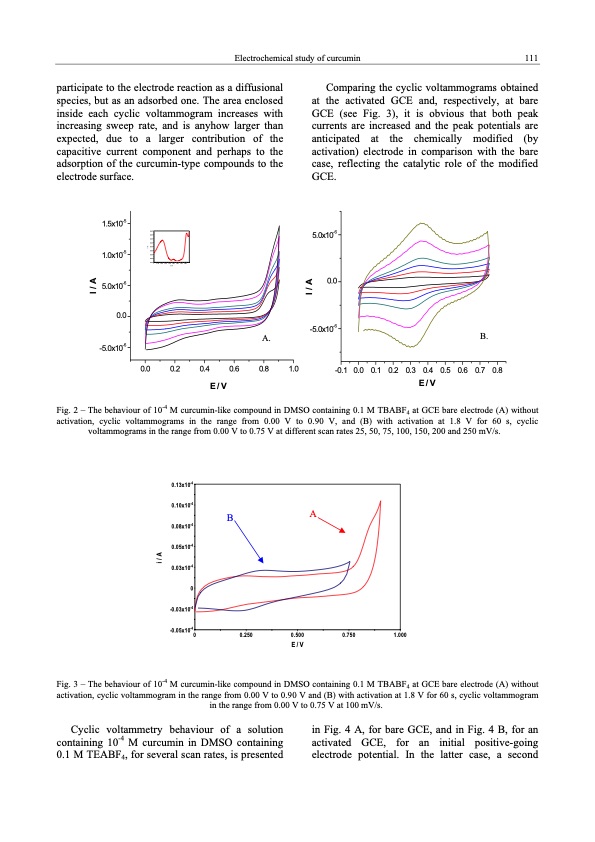
Electrochemical study of curcumin 111 participate to the electrode reaction as a diffusional species, but as an adsorbed one. The area enclosed inside each cyclic voltammogram increases with increasing sweep rate, and is anyhow larger than expected, due to a larger contribution of the capacitive current component and perhaps to the adsorption of the curcumin-type compounds to the electrode surface. Comparing the cyclic voltammograms obtained at the activated GCE and, respectively, at bare GCE (see Fig. 3), it is obvious that both peak currents are increased and the peak potentials are anticipated at the chemically modified (by activation) electrode in comparison with the bare case, reflecting the catalytic role of the modified GCE. 1.5x10-5 1.0x10-5 5.0x10-6 0.0 -5.0x10-6 A. 0.8 5.0x10-6 0.0 -5.0x10-6 B. 0.0 0.2 0.4 0.6 1.0 -0.1 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 E/V 0.60x10-5 0.55x10-5 0.50x10-5 0.45x10-5 0.40x10-5 0.35x10-5 0.30x10-5 0.25x10-5 0.20x10-50 0.100 0.200 0.300 0.400 0.500 E/V 0.600 0.700 0.800 Cyclic voltammetry behaviour of a solution containing 10-4 M curcumin in DMSO containing 0.1 M TEABF4, for several scan rates, is presented inFig.4A,forbareGCE,andinFig.4B,foran activated GCE, for an initial positive-going electrode potential. In the latter case, a second E/V Fig. 2 – The behaviour of 10-4 M curcumin-like compound in DMSO containing 0.1 M TBABF4 at GCE bare electrode (A) without activation, cyclic voltammograms in the range from 0.00 V to 0.90 V, and (B) with activation at 1.8 V for 60 s, cyclic voltammograms in the range from 0.00 V to 0.75 V at different scan rates 25, 50, 75, 100, 150, 200 and 250 mV/s. -4 0.13x10 -4 0.10x10 -4 0.08x10 -4 0.05x10 -4 0.03x10 0 -4 -0.03x10 -4 -0.05x10 B A 0 0.250 0.500 0.750 E/V 1.000 Fig. 3 – The behaviour of 10-4 M curcumin-like compound in DMSO containing 0.1 M TBABF4 at GCE bare electrode (A) without activation, cyclic voltammogram in the range from 0.00 V to 0.90 V and (B) with activation at 1.8 V for 60 s, cyclic voltammogram in the range from 0.00 V to 0.75 V at 100 mV/s. i/A I/A I/A i/APDF Image | ELECTROCHEMICAL STUDY OF CURCUMIN on carbon Electrode
PDF Search Title:
ELECTROCHEMICAL STUDY OF CURCUMIN on carbon ElectrodeOriginal File Name Searched:
electrochemical-curcumin.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |