
PDF Publication Title:
Text from PDF Page: 006
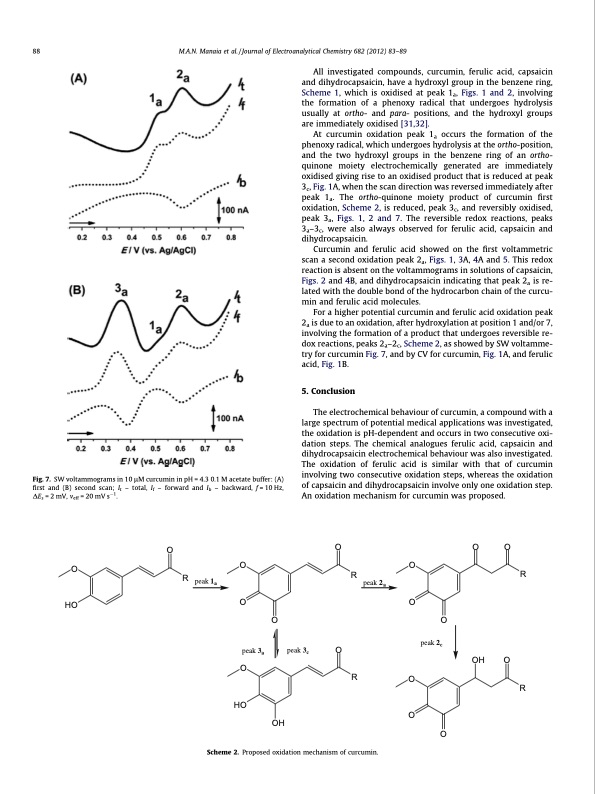
88 M.A.N. Manaia et al. / Journal of Electroanalytical Chemistry 682 (2012) 83–89 All investigated compounds, curcumin, ferulic acid, capsaicin and dihydrocapsaicin, have a hydroxyl group in the benzene ring, Scheme 1, which is oxidised at peak 1a, Figs. 1 and 2, involving the formation of a phenoxy radical that undergoes hydrolysis usually at ortho- and para- positions, and the hydroxyl groups are immediately oxidised [31,32]. At curcumin oxidation peak 1a occurs the formation of the phenoxy radical, which undergoes hydrolysis at the ortho-position, and the two hydroxyl groups in the benzene ring of an ortho- quinone moiety electrochemically generated are immediately oxidised giving rise to an oxidised product that is reduced at peak 3c, Fig. 1A, when the scan direction was reversed immediately after peak 1a. The ortho-quinone moiety product of curcumin first oxidation, Scheme 2, is reduced, peak 3c, and reversibly oxidised, peak 3a, Figs. 1, 2 and 7. The reversible redox reactions, peaks 3a–3c, were also always observed for ferulic acid, capsaicin and dihydrocapsaicin. Curcumin and ferulic acid showed on the first voltammetric scan a second oxidation peak 2a, Figs. 1, 3A, 4A and 5. This redox reaction is absent on the voltammograms in solutions of capsaicin, Figs. 2 and 4B, and dihydrocapsaicin indicating that peak 2a is re- lated with the double bond of the hydrocarbon chain of the curcu- min and ferulic acid molecules. For a higher potential curcumin and ferulic acid oxidation peak 2a is due to an oxidation, after hydroxylation at position 1 and/or 7, involving the formation of a product that undergoes reversible re- dox reactions, peaks 2a–2c, Scheme 2, as showed by SW voltamme- try for curcumin Fig. 7, and by CV for curcumin, Fig. 1A, and ferulic acid, Fig. 1B. 5. Conclusion The electrochemical behaviour of curcumin, a compound with a large spectrum of potential medical applications was investigated, the oxidation is pH-dependent and occurs in two consecutive oxi- dation steps. The chemical analogues ferulic acid, capsaicin and dihydrocapsaicin electrochemical behaviour was also investigated. The oxidation of ferulic acid is similar with that of curcumin involving two consecutive oxidation steps, whereas the oxidation of capsaicin and dihydrocapsaicin involve only one oxidation step. An oxidation mechanism for curcumin was proposed. Fig. 7. SW voltammograms in 10 lM curcumin in pH = 4.3 0.1 M acetate buffer: (A) first and (B) second scan; It – total, If – forward and Ib – backward, f = 10 Hz, DEs =2mV, veff =20mVs1. Scheme 2. Proposed oxidation mechanism of curcumin. O HO R O OOO OO R peak1 R a peak 2a OO peak 3a O HO OH peak 3c O peak 2c O O O OO R OH O RPDF Image | Guaicolic spices curcumin and capsaicin electrochemical oxidation
PDF Search Title:
Guaicolic spices curcumin and capsaicin electrochemical oxidationOriginal File Name Searched:
170-curcumin-JEAC.PDFDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |