
PDF Publication Title:
Text from PDF Page: 029
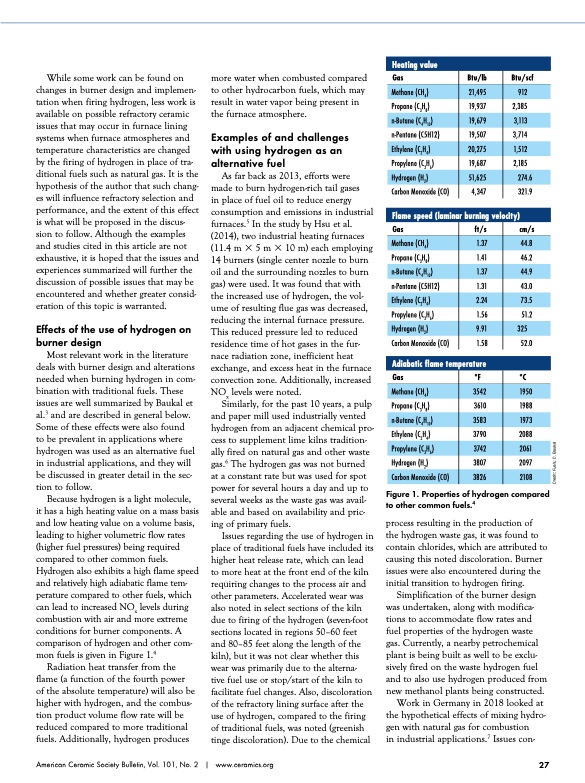
Heating value While some work can be found on changes in burner design and implemen- tation when firing hydrogen, less work is available on possible refractory ceramic issues that may occur in furnace lining systems when furnace atmospheres and temperature characteristics are changed by the firing of hydrogen in place of tra- ditional fuels such as natural gas. It is the hypothesis of the author that such chang- es will influence refractory selection and performance, and the extent of this effect is what will be proposed in the discus- sion to follow. Although the examples and studies cited in this article are not exhaustive, it is hoped that the issues and experiences summarized will further the discussion of possible issues that may be encountered and whether greater consid- eration of this topic is warranted. Effects of the use of hydrogen on burner design Most relevant work in the literature deals with burner design and alterations needed when burning hydrogen in com- bination with traditional fuels. These issues are well summarized by Baukal et al.3 and are described in general below. Some of these effects were also found to be prevalent in applications where hydrogen was used as an alternative fuel in industrial applications, and they will be discussed in greater detail in the sec- tion to follow. Because hydrogen is a light molecule, it has a high heating value on a mass basis and low heating value on a volume basis, leading to higher volumetric flow rates (higher fuel pressures) being required compared to other common fuels. Hydrogen also exhibits a high flame speed and relatively high adiabatic flame tem- perature compared to other fuels, which can lead to increased NOx levels during combustion with air and more extreme conditions for burner components. A comparison of hydrogen and other com- mon fuels is given in Figure 1.4 Radiation heat transfer from the flame (a function of the fourth power of the absolute temperature) will also be higher with hydrogen, and the combus- tion product volume flow rate will be reduced compared to more traditional fuels. Additionally, hydrogen produces more water when combusted compared Gas to other hydrocarbon fuels, which may Btu/scf 2,385 3,714 2,185 4,347 321.9 cm/s 46.2 43.0 51.2 1.58 52.0 °C 1988 2088 2097 Hydrogen (H2) Flame speed (laminar burning velocity) Methane (CH4) n-Butane (C4H10) Ethylene (C2H4) Hydrogen (H2) Methane (CH4) n-Butane (C4H10) Propylene (C3H6) Carbon Monoxide (CO) Btu/lb result in water vapor being present in the furnace atmosphere. Examples of and challenges with using hydrogen as an alternative fuel Propylene (C3H6) Propane (C3H8) Carbon Monoxide (CO) Gas Propane (C3H8) n-Pentane (C5H12) Propylene (C3H6) Carbon Monoxide (CO) Gas Propane (C3H8) Ethylene (C2H4) Hydrogen (H2) 21,495 51,625 ft/s 1.37 1.41 1.37 1.31 2.24 1.56 9.91 3542 3610 3583 3790 3742 3807 3826 912 n-Pentane (C5H12) As far back as 2013, efforts were made to burn hydrogen-rich tail gases in place of fuel oil to reduce energy consumption and emissions in industrial furnaces.5 In the study by Hsu et al. (2014), two industrial heating furnaces (11.4 m 3 5 m 3 10 m) each employing 14 burners (single center nozzle to burn oil and the surrounding nozzles to burn gas) were used. It was found that with the increased use of hydrogen, the vol- ume of resulting flue gas was decreased, reducing the internal furnace pressure. This reduced pressure led to reduced residence time of hot gases in the fur- nace radiation zone, inefficient heat exchange, and excess heat in the furnace convection zone. Additionally, increased NOx levels were noted. Similarly, for the past 10 years, a pulp and paper mill used industrially vented hydrogen from an adjacent chemical pro- cess to supplement lime kilns tradition- ally fired on natural gas and other waste gas.6 The hydrogen gas was not burned at a constant rate but was used for spot power for several hours a day and up to several weeks as the waste gas was avail- able and based on availability and pric- ing of primary fuels. Issues regarding the use of hydrogen in place of traditional fuels have included its higher heat release rate, which can lead to more heat at the front end of the kiln requiring changes to the process air and other parameters. Accelerated wear was also noted in select sections of the kiln due to firing of the hydrogen (seven-foot sections located in regions 50–60 feet and 80–85 feet along the length of the kiln), but it was not clear whether this wear was primarily due to the alterna- tive fuel use or stop/start of the kiln to facilitate fuel changes. Also, discoloration of the refractory lining surface after the use of hydrogen, compared to the firing of traditional fuels, was noted (greenish tinge discoloration). Due to the chemical Figure 1. Properties of hydrogen compared to other common fuels.4 process resulting in the production of the hydrogen waste gas, it was found to contain chlorides, which are attributed to causing this noted discoloration. Burner issues were also encountered during the initial transition to hydrogen firing. Simplification of the burner design was undertaken, along with modifica- tions to accommodate flow rates and fuel properties of the hydrogen waste gas. Currently, a nearby petrochemical plant is being built as well to be exclu- sively fired on the waste hydrogen fuel and to also use hydrogen produced from new methanol plants being constructed. Work in Germany in 2018 looked at the hypothetical effects of mixing hydro- gen with natural gas for combustion in industrial applications.7 Issues con- 274.6 44.8 44.9 73.5 325 Adiabatic flame temperature °F 1950 1973 2061 American Ceramic Society Bulletin, Vol. 101, No. 2 | www.ceramics.org 27 Methane (CH4) n-Butane (C4H10) Ethylene (C2H4) 19,937 19,679 19,507 20,275 19,687 3,113 1,512 2108 Credit: Fuels, C. BaukalPDF Image | hydrogen as an alternative fuel
PDF Search Title:
hydrogen as an alternative fuelOriginal File Name Searched:
March2022.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |