
PDF Publication Title:
Text from PDF Page: 035
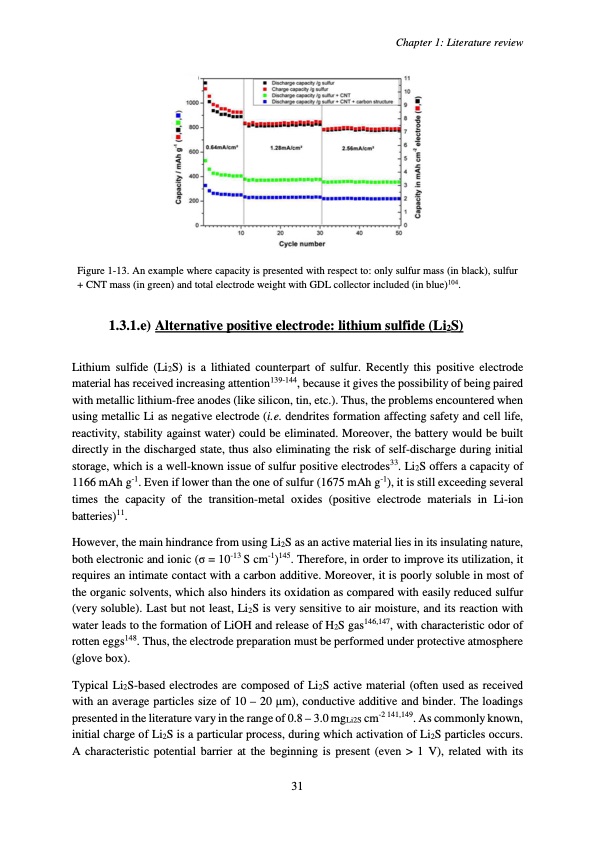
Figure 1-13. An example where capacity is presented with respect to: only sulfur mass (in black), sulfur + CNT mass (in green) and total electrode weight with GDL collector included (in blue)104. 1.3.1.e) Alternative positive electrode: lithium sulfide (Li2S) Lithium sulfide (Li2S) is a lithiated counterpart of sulfur. Recently this positive electrode material has received increasing attention139-144, because it gives the possibility of being paired with metallic lithium-free anodes (like silicon, tin, etc.). Thus, the problems encountered when using metallic Li as negative electrode (i.e. dendrites formation affecting safety and cell life, reactivity, stability against water) could be eliminated. Moreover, the battery would be built directly in the discharged state, thus also eliminating the risk of self-discharge during initial storage, which is a well-known issue of sulfur positive electrodes33. Li2S offers a capacity of 1166 mAh g-1. Even if lower than the one of sulfur (1675 mAh g-1), it is still exceeding several times the capacity of the transition-metal oxides (positive electrode materials in Li-ion batteries)11. However, the main hindrance from using Li2S as an active material lies in its insulating nature, both electronic and ionic (σ = 10-13 S cm-1)145. Therefore, in order to improve its utilization, it requires an intimate contact with a carbon additive. Moreover, it is poorly soluble in most of the organic solvents, which also hinders its oxidation as compared with easily reduced sulfur (very soluble). Last but not least, Li2S is very sensitive to air moisture, and its reaction with water leads to the formation of LiOH and release of H2S gas146,147, with characteristic odor of rotten eggs148. Thus, the electrode preparation must be performed under protective atmosphere (glove box). Typical Li2S-based electrodes are composed of Li2S active material (often used as received with an average particles size of 10 – 20 μm), conductive additive and binder. The loadings presented in the literature vary in the range of 0.8 – 3.0 mgLi2S cm-2 141,149. As commonly known, initial charge of Li2S is a particular process, during which activation of Li2S particles occurs. A characteristic potential barrier at the beginning is present (even > 1 V), related with its Chapter 1: Literature review 31PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |