
PDF Publication Title:
Text from PDF Page: 036
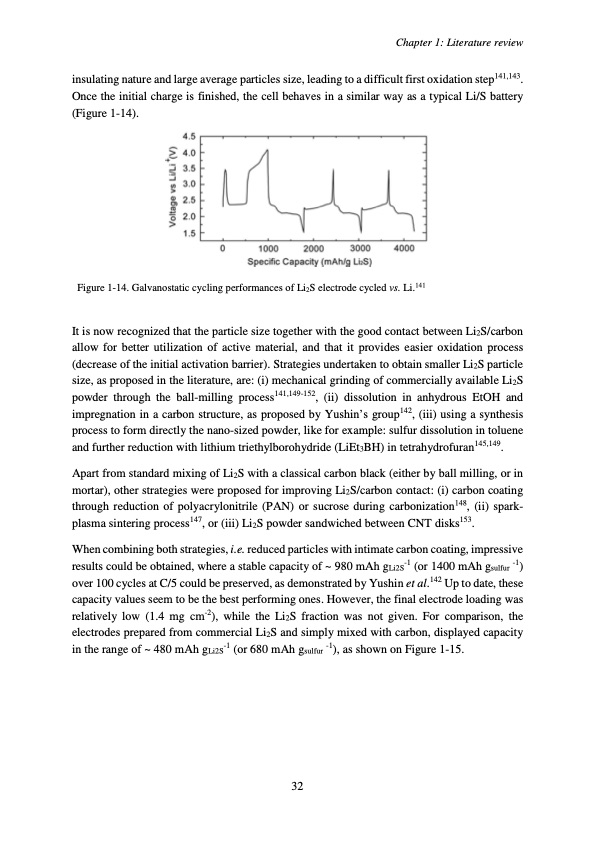
insulating nature and large average particles size, leading to a difficult first oxidation step141,143. Once the initial charge is finished, the cell behaves in a similar way as a typical Li/S battery (Figure 1-14). Figure 1-14. Galvanostatic cycling performances of Li2S electrode cycled vs. Li.141 It is now recognized that the particle size together with the good contact between Li2S/carbon allow for better utilization of active material, and that it provides easier oxidation process (decrease of the initial activation barrier). Strategies undertaken to obtain smaller Li2S particle size, as proposed in the literature, are: (i) mechanical grinding of commercially available Li2S powder through the ball-milling process141,149-152, (ii) dissolution in anhydrous EtOH and impregnation in a carbon structure, as proposed by Yushin’s group142, (iii) using a synthesis process to form directly the nano-sized powder, like for example: sulfur dissolution in toluene and further reduction with lithium triethylborohydride (LiEt3BH) in tetrahydrofuran145,149. Apart from standard mixing of Li2S with a classical carbon black (either by ball milling, or in mortar), other strategies were proposed for improving Li2S/carbon contact: (i) carbon coating through reduction of polyacrylonitrile (PAN) or sucrose during carbonization148, (ii) spark- plasma sintering process147, or (iii) Li2S powder sandwiched between CNT disks153. When combining both strategies, i.e. reduced particles with intimate carbon coating, impressive results could be obtained, where a stable capacity of ~ 980 mAh gLi2S-1 (or 1400 mAh gsulfur -1) over 100 cycles at C/5 could be preserved, as demonstrated by Yushin et al.142 Up to date, these capacity values seem to be the best performing ones. However, the final electrode loading was relatively low (1.4 mg cm-2), while the Li2S fraction was not given. For comparison, the electrodes prepared from commercial Li2S and simply mixed with carbon, displayed capacity in the range of ~ 480 mAh gLi2S-1 (or 680 mAh gsulfur -1), as shown on Figure 1-15. Chapter 1: Literature review 32PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |