
PDF Publication Title:
Text from PDF Page: 059
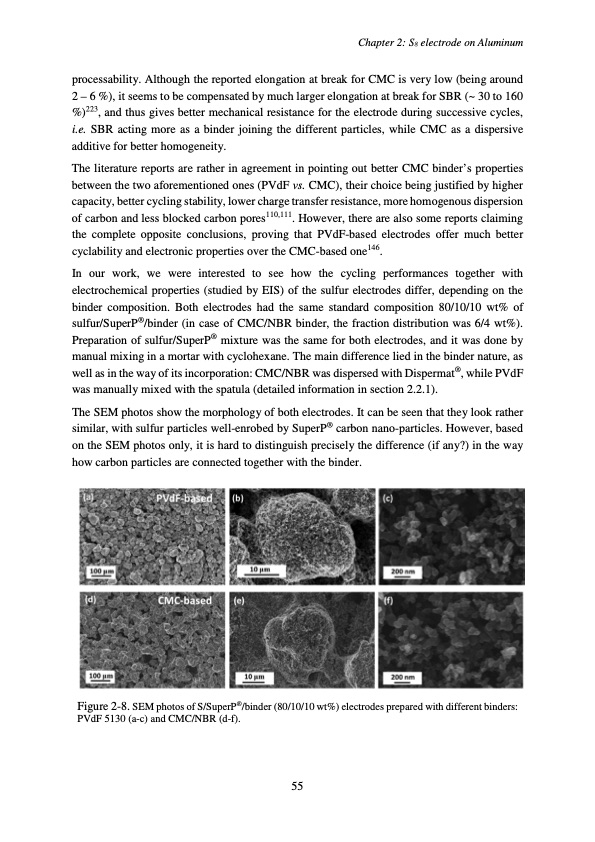
processability. Although the reported elongation at break for CMC is very low (being around 2 – 6 %), it seems to be compensated by much larger elongation at break for SBR (~ 30 to 160 %)223, and thus gives better mechanical resistance for the electrode during successive cycles, i.e. SBR acting more as a binder joining the different particles, while CMC as a dispersive additive for better homogeneity. The literature reports are rather in agreement in pointing out better CMC binder’s properties between the two aforementioned ones (PVdF vs. CMC), their choice being justified by higher capacity, better cycling stability, lower charge transfer resistance, more homogenous dispersion of carbon and less blocked carbon pores110,111. However, there are also some reports claiming the complete opposite conclusions, proving that PVdF-based electrodes offer much better cyclability and electronic properties over the CMC-based one146. In our work, we were interested to see how the cycling performances together with electrochemical properties (studied by EIS) of the sulfur electrodes differ, depending on the binder composition. Both electrodes had the same standard composition 80/10/10 wt% of sulfur/SuperP®/binder (in case of CMC/NBR binder, the fraction distribution was 6/4 wt%). Preparation of sulfur/SuperP® mixture was the same for both electrodes, and it was done by manual mixing in a mortar with cyclohexane. The main difference lied in the binder nature, as well as in the way of its incorporation: CMC/NBR was dispersed with Dispermat®, while PVdF was manually mixed with the spatula (detailed information in section 2.2.1). The SEM photos show the morphology of both electrodes. It can be seen that they look rather similar, with sulfur particles well-enrobed by SuperP® carbon nano-particles. However, based on the SEM photos only, it is hard to distinguish precisely the difference (if any?) in the way how carbon particles are connected together with the binder. Figure 2-8. SEM photos of S/SuperP®/binder (80/10/10 wt%) electrodes prepared with different binders: PVdF 5130 (a-c) and CMC/NBR (d-f). Chapter 2: S8 electrode on Aluminum 55PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |