
PDF Publication Title:
Text from PDF Page: 083
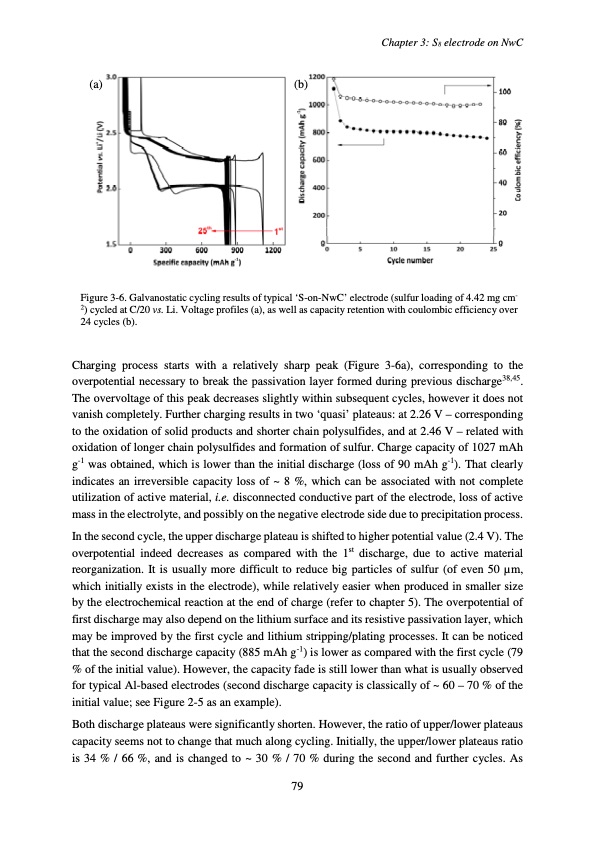
Chapter 3: S8 electrode on NwC (a) (b) Figure 3-6. Galvanostatic cycling results of typical ‘S-on-NwC’ electrode (sulfur loading of 4.42 mg cm- 2) cycled at C/20 vs. Li. Voltage profiles (a), as well as capacity retention with coulombic efficiency over 24 cycles (b). Charging process starts with a relatively sharp peak (Figure 3-6a), corresponding to the overpotential necessary to break the passivation layer formed during previous discharge38,45. The overvoltage of this peak decreases slightly within subsequent cycles, however it does not vanish completely. Further charging results in two ‘quasi’ plateaus: at 2.26 V – corresponding to the oxidation of solid products and shorter chain polysulfides, and at 2.46 V – related with oxidation of longer chain polysulfides and formation of sulfur. Charge capacity of 1027 mAh g-1 was obtained, which is lower than the initial discharge (loss of 90 mAh g-1). That clearly indicates an irreversible capacity loss of ~ 8 %, which can be associated with not complete utilization of active material, i.e. disconnected conductive part of the electrode, loss of active mass in the electrolyte, and possibly on the negative electrode side due to precipitation process. In the second cycle, the upper discharge plateau is shifted to higher potential value (2.4 V). The overpotential indeed decreases as compared with the 1st discharge, due to active material reorganization. It is usually more difficult to reduce big particles of sulfur (of even 50 μm, which initially exists in the electrode), while relatively easier when produced in smaller size by the electrochemical reaction at the end of charge (refer to chapter 5). The overpotential of first discharge may also depend on the lithium surface and its resistive passivation layer, which may be improved by the first cycle and lithium stripping/plating processes. It can be noticed that the second discharge capacity (885 mAh g-1) is lower as compared with the first cycle (79 % of the initial value). However, the capacity fade is still lower than what is usually observed for typical Al-based electrodes (second discharge capacity is classically of ~ 60 – 70 % of the initial value; see Figure 2-5 as an example). Both discharge plateaus were significantly shorten. However, the ratio of upper/lower plateaus capacity seems not to change that much along cycling. Initially, the upper/lower plateaus ratio is 34 % / 66 %, and is changed to ~ 30 % / 70 % during the second and further cycles. As 79PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |