
PDF Publication Title:
Text from PDF Page: 088
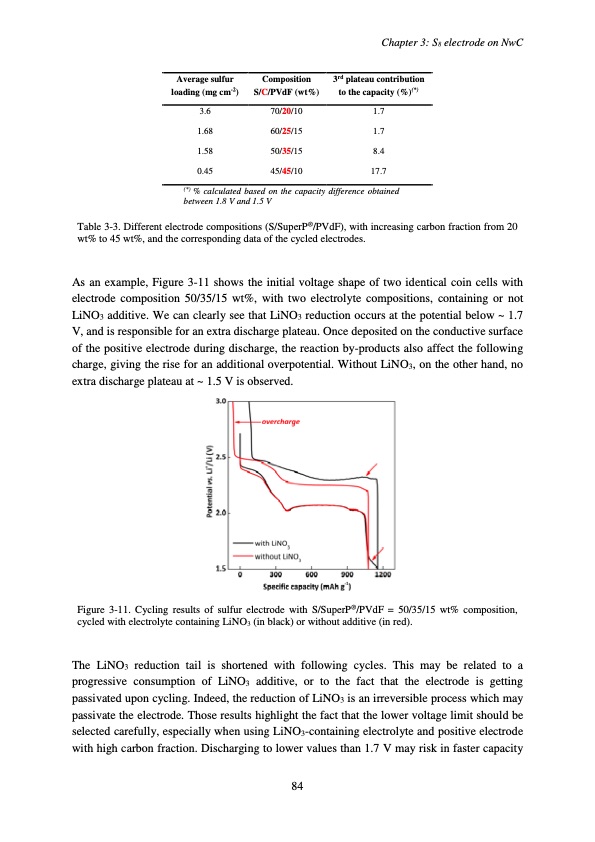
Chapter 3: S8 electrode on NwC Average sulfur loading (mg cm-2) 3.6 1.68 1.58 0.45 Composition 3rd plateau contribution S/C/PVdF (wt%) to the capacity (%)(*) 70/20/10 1.7 60/25/15 1.7 50/35/15 8.4 45/45/10 17.7 (*) % calculated based on the capacity difference obtained between 1.8 V and 1.5 V Table 3-3. Different electrode compositions (S/SuperP®/PVdF), with increasing carbon fraction from 20 wt% to 45 wt%, and the corresponding data of the cycled electrodes. As an example, Figure 3-11 shows the initial voltage shape of two identical coin cells with electrode composition 50/35/15 wt%, with two electrolyte compositions, containing or not LiNO3 additive. We can clearly see that LiNO3 reduction occurs at the potential below ~ 1.7 V, and is responsible for an extra discharge plateau. Once deposited on the conductive surface of the positive electrode during discharge, the reaction by-products also affect the following charge, giving the rise for an additional overpotential. Without LiNO3, on the other hand, no extra discharge plateau at ~ 1.5 V is observed. Figure 3-11. Cycling results of sulfur electrode with S/SuperP®/PVdF = 50/35/15 wt% composition, cycled with electrolyte containing LiNO3 (in black) or without additive (in red). The LiNO3 reduction tail is shortened with following cycles. This may be related to a progressive consumption of LiNO3 additive, or to the fact that the electrode is getting passivated upon cycling. Indeed, the reduction of LiNO3 is an irreversible process which may passivate the electrode. Those results highlight the fact that the lower voltage limit should be selected carefully, especially when using LiNO3-containing electrolyte and positive electrode with high carbon fraction. Discharging to lower values than 1.7 V may risk in faster capacity 84PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |