
PDF Publication Title:
Text from PDF Page: 092
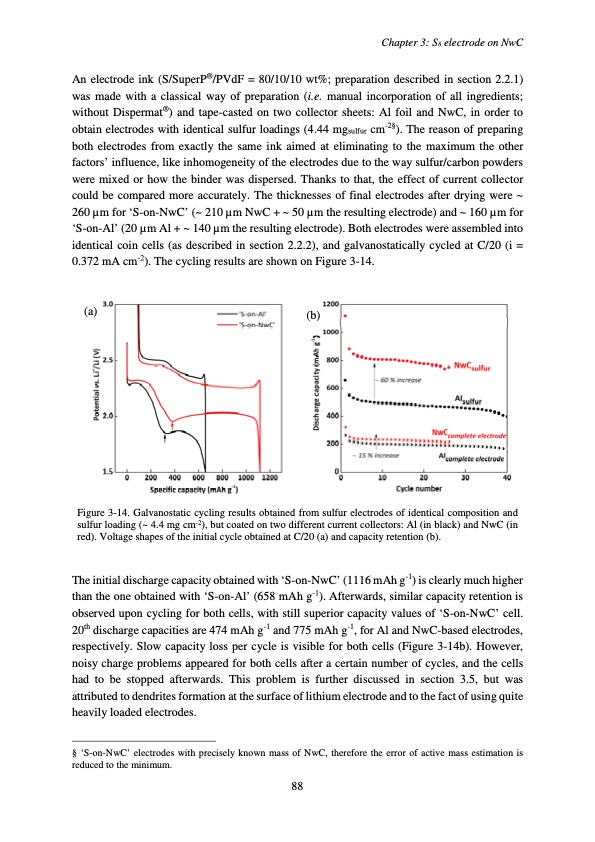
An electrode ink (S/SuperP®/PVdF = 80/10/10 wt%; preparation described in section 2.2.1) was made with a classical way of preparation (i.e. manual incorporation of all ingredients; without Dispermat®) and tape-casted on two collector sheets: Al foil and NwC, in order to obtain electrodes with identical sulfur loadings (4.44 mgsulfur cm-2§). The reason of preparing both electrodes from exactly the same ink aimed at eliminating to the maximum the other factors’ influence, like inhomogeneity of the electrodes due to the way sulfur/carbon powders were mixed or how the binder was dispersed. Thanks to that, the effect of current collector could be compared more accurately. The thicknesses of final electrodes after drying were ~ 260 μm for ‘S-on-NwC’ (~ 210 μm NwC + ~ 50 μm the resulting electrode) and ~ 160 μm for ‘S-on-Al’ (20 μm Al + ~ 140 μm the resulting electrode). Both electrodes were assembled into identical coin cells (as described in section 2.2.2), and galvanostatically cycled at C/20 (i = 0.372 mA cm-2). The cycling results are shown on Figure 3-14. Chapter 3: S8 electrode on NwC (a) (b) Figure 3-14. Galvanostatic cycling results obtained from sulfur electrodes of identical composition and sulfur loading (~ 4.4 mg cm-2), but coated on two different current collectors: Al (in black) and NwC (in red). Voltage shapes of the initial cycle obtained at C/20 (a) and capacity retention (b). The initial discharge capacity obtained with ‘S-on-NwC’ (1116 mAh g-1) is clearly much higher than the one obtained with ‘S-on-Al’ (658 mAh g-1). Afterwards, similar capacity retention is observed upon cycling for both cells, with still superior capacity values of ‘S-on-NwC’ cell. 20th discharge capacities are 474 mAh g-1 and 775 mAh g-1, for Al and NwC-based electrodes, respectively. Slow capacity loss per cycle is visible for both cells (Figure 3-14b). However, noisy charge problems appeared for both cells after a certain number of cycles, and the cells had to be stopped afterwards. This problem is further discussed in section 3.5, but was attributed to dendrites formation at the surface of lithium electrode and to the fact of using quite heavily loaded electrodes. § ‘S-on-NwC’ electrodes with precisely known mass of NwC, therefore the error of active mass estimation is reduced to the minimum. 88PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |