
PDF Publication Title:
Text from PDF Page: 096
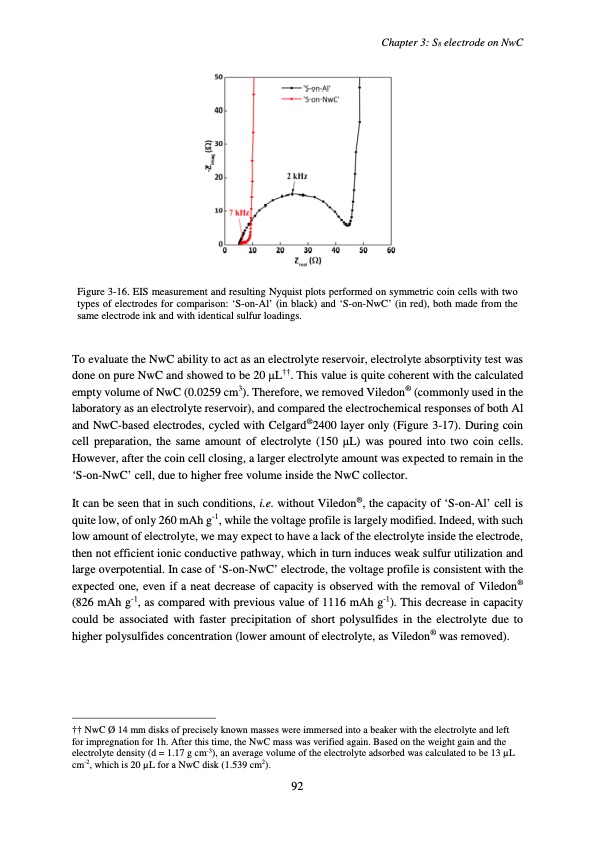
Chapter 3: S8 electrode on NwC Figure 3-16. EIS measurement and resulting Nyquist plots performed on symmetric coin cells with two types of electrodes for comparison: ‘S-on-Al’ (in black) and ‘S-on-NwC’ (in red), both made from the same electrode ink and with identical sulfur loadings. To evaluate the NwC ability to act as an electrolyte reservoir, electrolyte absorptivity test was done on pure NwC and showed to be 20 μL††. This value is quite coherent with the calculated empty volume of NwC (0.0259 cm3). Therefore, we removed Viledon® (commonly used in the laboratory as an electrolyte reservoir), and compared the electrochemical responses of both Al and NwC-based electrodes, cycled with Celgard®2400 layer only (Figure 3-17). During coin cell preparation, the same amount of electrolyte (150 μL) was poured into two coin cells. However, after the coin cell closing, a larger electrolyte amount was expected to remain in the ‘S-on-NwC’ cell, due to higher free volume inside the NwC collector. It can be seen that in such conditions, i.e. without Viledon®, the capacity of ‘S-on-Al’ cell is quite low, of only 260 mAh g-1, while the voltage profile is largely modified. Indeed, with such low amount of electrolyte, we may expect to have a lack of the electrolyte inside the electrode, then not efficient ionic conductive pathway, which in turn induces weak sulfur utilization and large overpotential. In case of ‘S-on-NwC’ electrode, the voltage profile is consistent with the expected one, even if a neat decrease of capacity is observed with the removal of Viledon® (826 mAh g-1, as compared with previous value of 1116 mAh g-1). This decrease in capacity could be associated with faster precipitation of short polysulfides in the electrolyte due to higher polysulfides concentration (lower amount of electrolyte, as Viledon® was removed). †† NwC Ø 14 mm disks of precisely known masses were immersed into a beaker with the electrolyte and left for impregnation for 1h. After this time, the NwC mass was verified again. Based on the weight gain and the electrolyte density (d = 1.17 g cm-3), an average volume of the electrolyte adsorbed was calculated to be 13 μL cm-2, which is 20 μL for a NwC disk (1.539 cm2). 92PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |