
PDF Publication Title:
Text from PDF Page: 004
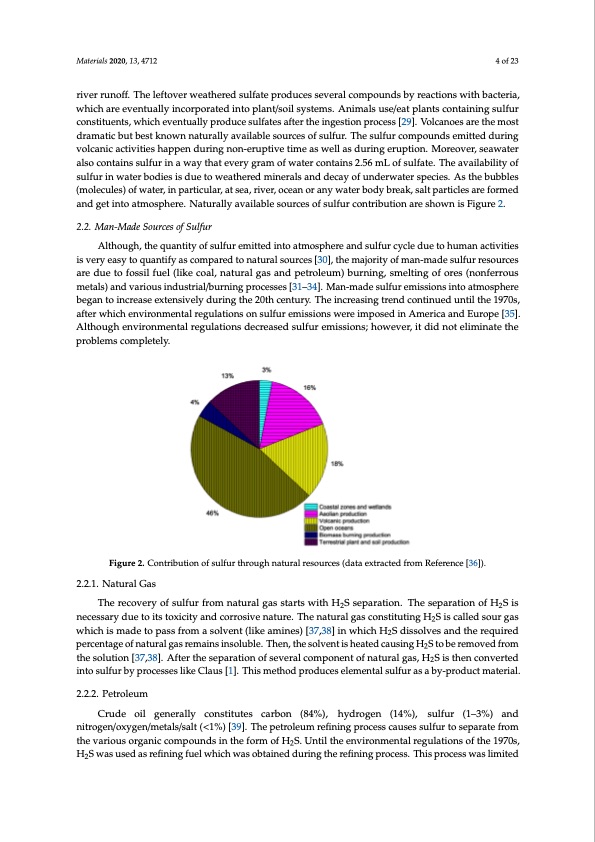
Materials 2020, 13, 4712 4 of 23 river runoff. The leftover weathered sulfate produces several compounds by reactions with bacteria, which are eventually incorporated into plant/soil systems. Animals use/eat plants containing sulfur constituents, which eventually produce sulfates after the ingestion process [29]. Volcanoes are the most dramatic but best known naturally available sources of sulfur. The sulfur compounds emitted during volcanic activities happen during non-eruptive time as well as during eruption. Moreover, seawater also contains sulfur in a way that every gram of water contains 2.56 mL of sulfate. The availability of sulfur in water bodies is due to weathered minerals and decay of underwater species. As the bubbles (molecules) of water, in particular, at sea, river, ocean or any water body break, salt particles are formed and get into atmosphere. Naturally available sources of sulfur contribution are shown is Figure 2. 2.2. Man-Made Sources of Sulfur Although, the quantity of sulfur emitted into atmosphere and sulfur cycle due to human activities is very easy to quantify as compared to natural sources [30], the majority of man-made sulfur resources are due to fossil fuel (like coal, natural gas and petroleum) burning, smelting of ores (nonferrous metals) and various industrial/burning processes [31–34]. Man-made sulfur emissions into atmosphere began to increase extensively during the 20th century. The increasing trend continued until the 1970s, after which environmental regulations on sulfur emissions were imposed in America and Europe [35]. Although environmental regulations decreased sulfur emissions; however, it did not eliminate the problems completely. Figure 2. Contribution of sulfur through natural resources (data extracted from Reference [36]). 2.2.1. Natural Gas The recovery of sulfur from natural gas starts with H2S separation. The separation of H2S is necessary due to its toxicity and corrosive nature. The natural gas constituting H2S is called sour gas which is made to pass from a solvent (like amines) [37,38] in which H2S dissolves and the required percentage of natural gas remains insoluble. Then, the solvent is heated causing H2S to be removed from the solution [37,38]. After the separation of several component of natural gas, H2S is then converted into sulfur by processes like Claus [1]. This method produces elemental sulfur as a by-product material. 2.2.2. Petroleum Crude oil generally constitutes carbon (84%), hydrogen (14%), sulfur (1–3%) and nitrogen/oxygen/metals/salt (<1%) [39]. The petroleum refining process causes sulfur to separate from the various organic compounds in the form of H2S. Until the environmental regulations of the 1970s, H2S was used as refining fuel which was obtained during the refining process. This process was limitedPDF Image | Critical Review on the Properties and Applications of Sulfur-Based Concrete
PDF Search Title:
Critical Review on the Properties and Applications of Sulfur-Based ConcreteOriginal File Name Searched:
materials-13-04712.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |