
PDF Publication Title:
Text from PDF Page: 007
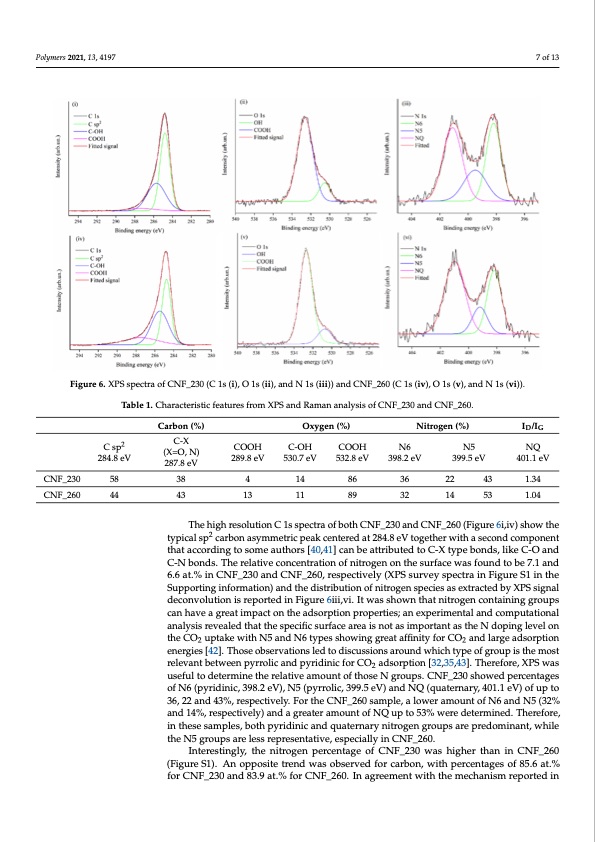
nary, 401.1 eV) of up to 36, 22 and 43%, respectively. For the CNF_260 sample, a lowe % n n ) e n s d 0 n h n Q y amount of N6 and N5 (32% and 14%, respectively) and a greater amount of NQ up to 53 were determined. Therefore, in these samples, both pyridinic and quaternary nitroge groups are predominant, while the N5 groups are less representative, especially i Polymers 2021, 13, 4197 7 of 13 CNF_260. Figure 6. XPS spectra of CNF_230 (C 1s (i), O 1s (ii), and N 1s (iii)) and CNF_260 (C 1s (iv), O 1s (v), and N 1s (vi)). Figure 6. XPS spectra of CNF_230 (C 1s (i), O 1s (ii), and N 1s (iii)) and CNF_260 (C 1s (iv), O 1s (v and N 1s (vi)T)a.ble 1. Characteristic features from XPS and Raman analysis of CNF_230 and CNF_260. Carbon (%) Oxygen (%) Nitrogen (%) ID/IG Interestingly, the nitrogen percentage of CNF_230 was higher than in CNF_260 (Fig C sp2 C-X COOH C-OH COOH N6 N5 NQ (X=O, N) ure S1).28A4.8neVopposite trend w28a9s.8eoVbse5r3v0.e7deVfor53c2a.8rebVon,3w98.i2tehV perce39n9.t5aegVes of40815.1.e6Vat.% fo 287.8 eV CNF_230 and 83.9 at.% for CNF_260. In agreement with the mechanism reported in Figur CNF_230 58 38 4 14 86 36 22 43 1.34 3, we hypothesized that the higher stabilization temperature improved aromatizatio CNF_260 44 43 13 11 89 32 14 53 1.04 through nitrile loss (Figure 3iv). The lower content of carbon in the CNF_260 sample wa The high resolution C 1s spectra of both CNF_230 and CNF_260 (Figure 6i,iv) show the reasonably due to the higher oxidation induced by the increment of the stabilization tem typical sp2 carbon asymmetric peak centered at 284.8 eV together with a second component perature as proven by the higher content of oxygen compared to CNF_230 (7.5 at.% an that according to some authors [40,41] can be attributed to C-X type bonds, like C-O and C-N bonds. The relative concentration of nitrogen on the surface was found to be 7.1 and 6.1 at.% respectively obtained by the XPS analysis). 6.6 at.% in CNF_230 and CNF_260, respectively (XPS survey spectra in Figure S1 in the Supporting information) and the distribution of nitrogen species as extracted by XPS signal 3.2. CO2 AdsorptiondPeceornfvoorlmutaioncies reported in Figure 6iii,vi. It was shown that nitrogen containing groups can have a great impact on the adsorption properties; an experimental and computational The CO2 adsorption performance was analyzed by using a volumetric analyzer at 2 analysis revealed that the specific surface area is not as important as the N doping level on theCO uptakewithN5andN6typesshowinggreataffinityforCO andlargeadsorption °C (see Figure 7i,ii). CN2F_230 and CNF_260 samples showed a ve2 ry similar adsorptio energies [42]. Those observations led to discussions around which type of group is the most behavior, but with better CO2 uptake for the latter, i.e., 4.16 mmol g−1 (18.3 wt.%) wit relevant between pyrrolic and pyridinic for CO2 adsorption [32,35,43]. Therefore, XPS was −1 respect to 2.75 mmuoslefgul to(1de2tewrmti.n%e t)heorfelCatiNveFa_m2o3u0nt soaf tmhopseleN.gArotupas.lCoNwF_p23r0eshsouwreed ptherecesnetatgweso sam of N6 (pyridinic, 398.2 eV), N5 (pyrrolic, 399.5 eV) and NQ (quaternary, 401.1 eV) of up to ples showed a very similar adsorption, while at a higher pressure the CO2 uptake o 36, 22 and 43%, respectively. For the CNF_260 sample, a lower amount of N6 and N5 (32% CNF_260 increased quickly. Some results on control samples are reported in the Supple and 14%, respectively) and a greater amount of NQ up to 53% were determined. Therefore, in these samples, both pyridinic and quaternary nitrogen groups are predominant, while mentary Information file. An “as spun” (not stabilized) sample (AS) was not efficient i the N5 groups are less representative, especially in CNF_260. CO2 adsorption (0.3 mmol g−1 of adsorbed gas) due to inadequate/absent carbon fiber for Interestingly, the nitrogen percentage of CNF_230 was higher than in CNF_260 (Figure S1). An opposite trend was observed for carbon, with percentages of 85.6 at.% mation, no porous structure, and likely no residual N groups of the kind N6, N5 and N for CNF_230 and 83.9 at.% for CNF_260. In agreement with the mechanism reported in The stabilized but not carbonized sample (STAB) showed a modest increment in CO2 ad sorption compared with the AS sample. The histogram reported in SI (Figure S3) clearlPDF Image | Effect of Thermal Stabilization on PAN-Derived Electrospun Carbon Nanofibers
PDF Search Title:
Effect of Thermal Stabilization on PAN-Derived Electrospun Carbon NanofibersOriginal File Name Searched:
polymers-13-04197-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |