
PDF Publication Title:
Text from PDF Page: 006
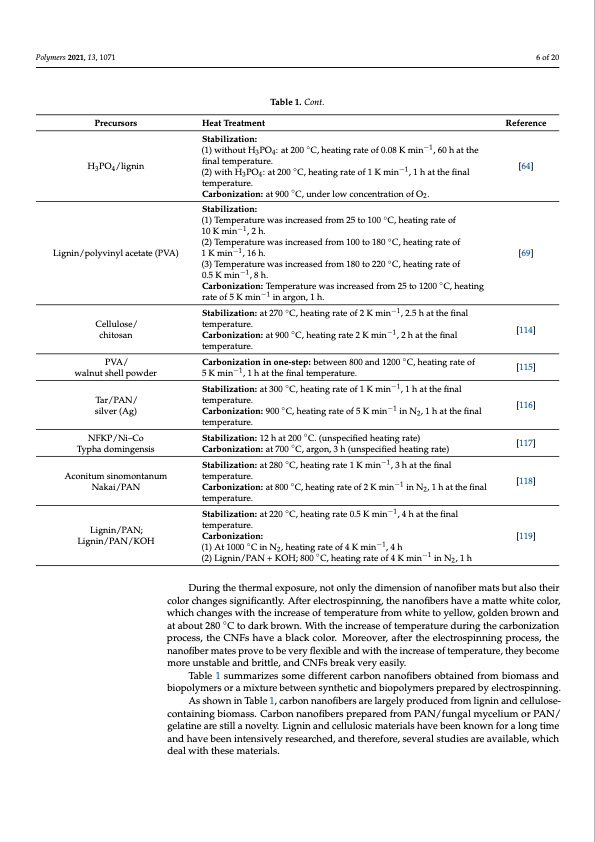
Polymers 2021, 13, 1071 6 of 20 Table 1. Cont. Precursors H3PO4/lignin Lignin/polyvinyl acetate (PVA) Cellulose/ chitosan PVA/ walnut shell powder Tar/PAN/ silver (Ag) NFKP/Ni–Co Typha domingensis Aconitum sinomontanum Nakai/PAN Lignin/PAN; Lignin/PAN/KOH Heat Treatment Stabilization: Reference [64] (1) without H3PO4: at 200 ◦C, heating rate of 0.08 K min−1, 60 h at the final temperature. ◦ (2)withH3PO4: at200 temperature. Carbonization: at 900 ◦C, under low concentration of O2. Stabilization: C,heatingrateof1Kmin −1 ,1hatthefinal (1) Temperature was increased from 25 to 100 ◦C, heating rate of 10 K min−1, 2 h. (2) Temperature was increased from 100 to 180 ◦C, heating rate of 1Kmin−1,16h. [69] (3) Temperature was increased from 180 to 220 ◦C, heating rate of 0.5 K min−1, 8 h. Carbonization: Temperature was increased from 25 to 1200 ◦C, heating rate of 5 K min−1 in argon, 1 h. Stabilization: at 270 ◦C, heating rate of 2 K min−1, 2.5 h at the final temperature. Carbonization: at 900 ◦C, heating rate 2 K min−1, 2 h at the final temperature. Carbonization in one-step: between 800 and 1200 ◦C, heating rate of 5 K min−1, 1 h at the final temperature. Stabilization: at 300 ◦C, heating rate of 1 K min−1, 1 h at the final temperature. Carbonization: 900 ◦C, heating rate of 5 K min−1 in N2, 1 h at the final temperature. Stabilization: 12 h at 200 ◦C. (unspecified heating rate) Carbonization: at 700 ◦C, argon, 3 h (unspecified heating rate) Stabilization: at 280 ◦C, heating rate 1 K min−1, 3 h at the final temperature. Carbonization: at 800 ◦C, heating rate of 2 K min−1 in N2, 1 h at the final temperature. Stabilization: at 220 ◦C, heating rate 0.5 K min−1, 4 h at the final temperature. Carbonization: (1) At 1000 ◦C in N2, heating rate of 4 K min−1, 4 h (2) Lignin/PAN + KOH; 800 ◦C, heating rate of 4 K min−1 in N2, 1 h [114] [115] [116] [117] [118] [119] During the thermal exposure, not only the dimension of nanofiber mats but also their color changes significantly. After electrospinning, the nanofibers have a matte white color, which changes with the increase of temperature from white to yellow, golden brown and at about 280 ◦C to dark brown. With the increase of temperature during the carbonization process, the CNFs have a black color. Moreover, after the electrospinning process, the nanofiber mates prove to be very flexible and with the increase of temperature, they become more unstable and brittle, and CNFs break very easily. Table 1 summarizes some different carbon nanofibers obtained from biomass and biopolymers or a mixture between synthetic and biopolymers prepared by electrospinning. As shown in Table 1, carbon nanofibers are largely produced from lignin and cellulose- containing biomass. Carbon nanofibers prepared from PAN/fungal mycelium or PAN/ gelatine are still a novelty. Lignin and cellulosic materials have been known for a long time and have been intensively researched, and therefore, several studies are available, which deal with these materials.PDF Image | Electrospun Carbon Nanofibers from Biomass and Biomass Blends
PDF Search Title:
Electrospun Carbon Nanofibers from Biomass and Biomass BlendsOriginal File Name Searched:
88145ac687f0c36c7096f236fabe2cba856c.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |