
PDF Publication Title:
Text from PDF Page: 005
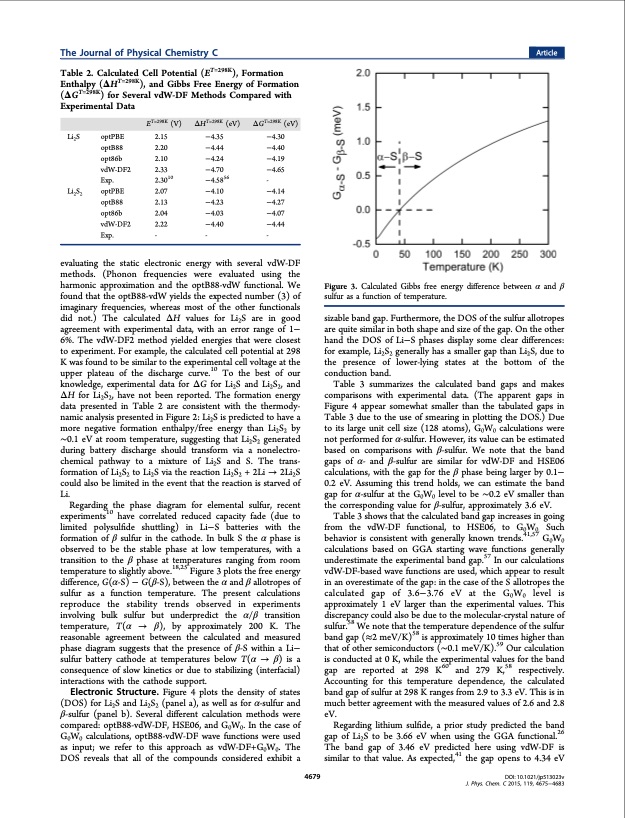
The Journal of Physical Chemistry C Table 2. Calculated Cell Potential (ET=298K), Formation Enthalpy (ΔHT=298K), and Gibbs Free Energy of Formation (ΔGT=298K) for Several vdW-DF Methods Compared with Experimental Data Article Li2S optPBE optB88 opt86b vdW-DF2 Exp. Li2S2 optPBE optB88 opt86b vdW-DF2 Exp. ET=298K (V) 2.15 2.20 2.10 2.33 2.3010 2.07 2.13 2.04 2.22 - ΔHT=298K (eV) −4.35 −4.44 −4.24 −4.70 −4.5856 −4.10 −4.23 −4.03 −4.40 - ΔGT=298K (eV) −4.30 −4.40 −4.19 −4.65 - −4.14 −4.27 −4.07 −4.44 - evaluating the static electronic energy with several vdW-DF methods. (Phonon frequencies were evaluated using the harmonic approximation and the optB88-vdW functional. We found that the optB88-vdW yields the expected number (3) of imaginary frequencies, whereas most of the other functionals did not.) The calculated ΔH values for Li2S are in good agreement with experimental data, with an error range of 1− 6%. The vdW-DF2 method yielded energies that were closest to experiment. For example, the calculated cell potential at 298 K was found to be similar to the experimental cell voltage at the upper plateau of the discharge curve.10 To the best of our knowledge, experimental data for ΔG for Li2S and Li2S2, and ΔH for Li2S2, have not been reported. The formation energy data presented in Table 2 are consistent with the thermody- namic analysis presented in Figure 2: Li2S is predicted to have a more negative formation enthalpy/free energy than Li2S2 by ∼0.1 eV at room temperature, suggesting that Li2S2 generated during battery discharge should transform via a nonelectro- chemical pathway to a mixture of Li2S and S. The trans- formation of Li2S2 to Li2S via the reaction Li2S2 + 2Li → 2Li2S could also be limited in the event that the reaction is starved of Li. Regarding the phase diagram for elemental sulfur, recent experiments10 have correlated reduced capacity fade (due to limited polysulfide shuttling) in Li−S batteries with the formation of β sulfur in the cathode. In bulk S the α phase is observed to be the stable phase at low temperatures, with a transition to the β phase at temperatures ranging from room temperature to slightly above.18,25 Figure 3 plots the free energy difference, G(α-S) − G(β-S), between the α and β allotropes of sulfur as a function temperature. The present calculations reproduce the stability trends observed in experiments involving bulk sulfur but underpredict the α/β transition temperature, T(α → β), by approximately 200 K. The reasonable agreement between the calculated and measured phase diagram suggests that the presence of β-S within a Li− sulfur battery cathode at temperatures below T(α → β) is a consequence of slow kinetics or due to stabilizing (interfacial) interactions with the cathode support. Electronic Structure. Figure 4 plots the density of states (DOS) for Li2S and Li2S2 (panel a), as well as for α-sulfur and β-sulfur (panel b). Several different calculation methods were compared: optB88-vdW-DF, HSE06, and G0W0. In the case of G0W0 calculations, optB88-vdW-DF wave functions were used as input; we refer to this approach as vdW-DF+G0W0. The DOS reveals that all of the compounds considered exhibit a 4679 Figure 3. Calculated Gibbs free energy difference between α and β sulfur as a function of temperature. sizable band gap. Furthermore, the DOS of the sulfur allotropes are quite similar in both shape and size of the gap. On the other hand the DOS of Li−S phases display some clear differences: for example, Li2S2 generally has a smaller gap than Li2S, due to the presence of lower-lying states at the bottom of the conduction band. Table 3 summarizes the calculated band gaps and makes comparisons with experimental data. (The apparent gaps in Figure 4 appear somewhat smaller than the tabulated gaps in Table 3 due to the use of smearing in plotting the DOS.) Due to its large unit cell size (128 atoms), G0W0 calculations were not performed for α-sulfur. However, its value can be estimated based on comparisons with β-sulfur. We note that the band gaps of α- and β-sulfur are similar for vdW-DF and HSE06 calculations, with the gap for the β phase being larger by 0.1− 0.2 eV. Assuming this trend holds, we can estimate the band gap for α-sulfur at the G0W0 level to be ∼0.2 eV smaller than the corresponding value for β-sulfur, approximately 3.6 eV. Table 3 shows that the calculated band gap increases in going from the vdW-DF functional, to HSE06, to G0W0. Such behavior is consistent with generally known trends.41,57 G0W0 calculations based on GGA starting wave functions generally underestimate the experimental band gap.57 In our calculations vdW-DF-based wave functions are used, which appear to result in an overestimate of the gap: in the case of the S allotropes the calculated gap of 3.6−3.76 eV at the G0W0 level is approximately 1 eV larger than the experimental values. This discrepancy could also be due to the molecular-crystal nature of sulfur.58 We note that the temperature dependence of the sulfur band gap (≈2 meV/K)58 is approximately 10 times higher than that of other semiconductors (∼0.1 meV/K).59 Our calculation is conducted at 0 K, while the experimental values for the band gap are reported at 298 K60 and 279 K,58 respectively. Accounting for this temperature dependence, the calculated band gap of sulfur at 298 K ranges from 2.9 to 3.3 eV. This is in much better agreement with the measured values of 2.6 and 2.8 eV. Regarding lithium sulfide, a prior study predicted the band gap of Li2S to be 3.66 eV when using the GGA functional.26 The band gap of 3.46 eV predicted here using vdW-DF is similar to that value. As expected,41 the gap opens to 4.34 eV DOI: 10.1021/jp513023v J. Phys. Chem. C 2015, 119, 4675−4683PDF Image | First-Principles Study of Redox End Members in Lithium Sulfur
PDF Search Title:
First-Principles Study of Redox End Members in Lithium SulfurOriginal File Name Searched:
JPCC_Li-S.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |