
PDF Publication Title:
Text from PDF Page: 056
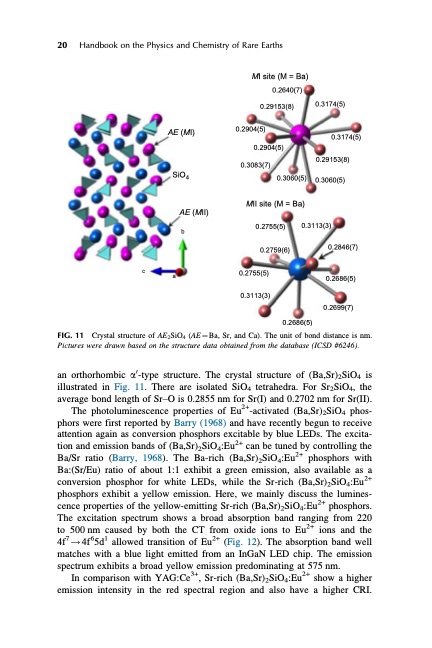
20 Handbook on the Physics and Chemistry of Rare Earths MI site (M = Ba) AE (MI) SiO4 AE (MII) b c a Crystal structure of AE2SiO4 (AE1⁄4Ba, Sr, and Ca). The unit of bond distance is nm. Pictures were drawn based on the structure data obtained from the database (ICSD #6246). an orthorhombic a0-type structure. The crystal structure of (Ba,Sr)2SiO4 is illustrated in Fig. 11. There are isolated SiO4 tetrahedra. For Sr2SiO4, the average bond length of Sr–O is 0.2855 nm for Sr(I) and 0.2702 nm for Sr(II). The photoluminescence properties of Eu2+-activated (Ba,Sr)2SiO4 phos- phors were first reported by Barry (1968) and have recently begun to receive attention again as conversion phosphors excitable by blue LEDs. The excita- tion and emission bands of (Ba,Sr)2SiO4:Eu2+ can be tuned by controlling the Ba/Sr ratio (Barry, 1968). The Ba-rich (Ba,Sr)2SiO4:Eu2+ phosphors with Ba:(Sr/Eu) ratio of about 1:1 exhibit a green emission, also available as a conversion phosphor for white LEDs, while the Sr-rich (Ba,Sr)2SiO4:Eu2+ phosphors exhibit a yellow emission. Here, we mainly discuss the lumines- cence properties of the yellow-emitting Sr-rich (Ba,Sr)2SiO4:Eu2+ phosphors. The excitation spectrum shows a broad absorption band ranging from 220 to 500 nm caused by both the CT from oxide ions to Eu2+ ions and the 4f7!4f65d1 allowed transition of Eu2+ (Fig. 12). The absorption band well matches with a blue light emitted from an InGaN LED chip. The emission spectrum exhibits a broad yellow emission predominating at 575 nm. In comparison with YAG:Ce3+, Sr-rich (Ba,Sr)2SiO4:Eu2+ show a higher emission intensity in the red spectral region and also have a higher CRI. FIG. 11 0.2640(7) 0.29153(8) 0.2904(5) 0.2904(5) 0.3083(7) 0.3174(5) 0.3174(5) 0.29153(8) 0.3060(5) 0.3060(5) MII site (M = Ba) 0.2755(5) 0.3113(3) 0.2755(5) 0.2759(6) 0.3113(3) 0.2846(7) 0.2686(5) 0.2699(7) 0.2686(5)PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |