
PDF Publication Title:
Text from PDF Page: 064
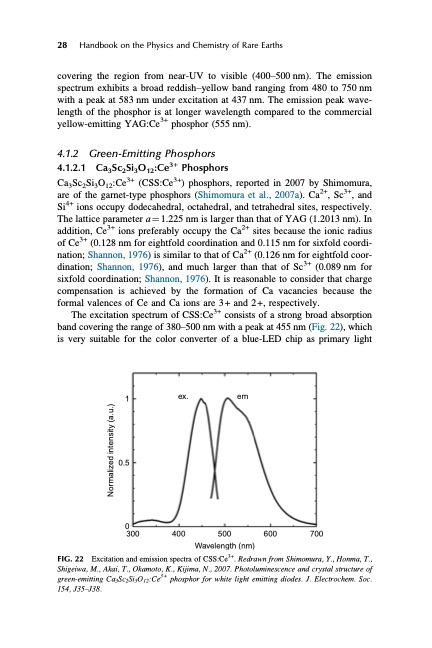
28 Handbook on the Physics and Chemistry of Rare Earths covering the region from near-UV to visible (400–500 nm). The emission spectrum exhibits a broad reddish–yellow band ranging from 480 to 750 nm with a peak at 583 nm under excitation at 437 nm. The emission peak wave- length of the phosphor is at longer wavelength compared to the commercial yellow-emitting YAG:Ce3+ phosphor (555 nm). 4.1.2 Green-Emitting Phosphors 4.1.2.1 Ca3Sc2Si3O12:Ce3+ Phosphors Ca3Sc2Si3O12:Ce3+ (CSS:Ce3+) phosphors, reported in 2007 by Shimomura, are of the garnet-type phosphors (Shimomura et al., 2007a). Ca2+, Sc3+, and Si4+ ions occupy dodecahedral, octahedral, and tetrahedral sites, respectively. The lattice parameter a 1⁄4 1.225 nm is larger than that of YAG (1.2013 nm). In addition, Ce3+ ions preferably occupy the Ca2+ sites because the ionic radius of Ce3+ (0.128 nm for eightfold coordination and 0.115 nm for sixfold coordi- nation; Shannon, 1976) is similar to that of Ca2+ (0.126 nm for eightfold coor- dination; Shannon, 1976), and much larger than that of Sc3+ (0.089 nm for sixfold coordination; Shannon, 1976). It is reasonable to consider that charge compensation is achieved by the formation of Ca vacancies because the formal valences of Ce and Ca ions are 3+ and 2+, respectively. The excitation spectrum of CSS:Ce3+ consists of a strong broad absorption band covering the range of 380–500 nm with a peak at 455 nm (Fig. 22), which is very suitable for the color converter of a blue-LED chip as primary light 1 0.5 ex. em 0 300 400 500 600 700 Wavelength (nm) Excitation and emission spectra of CSS:Ce3+. Redrawn from Shimomura, Y., Honma, T., Shigeiwa, M., Akai, T., Okamoto, K., Kijima, N., 2007. Photoluminescence and crystal structure of green-emitting Ca3Sc2Si3O12:Ce3+ phosphor for white light emitting diodes. J. Electrochem. Soc. 154, J35–J38. FIG. 22 Normalized intensity (a.u.)PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |