
PDF Publication Title:
Text from PDF Page: 066
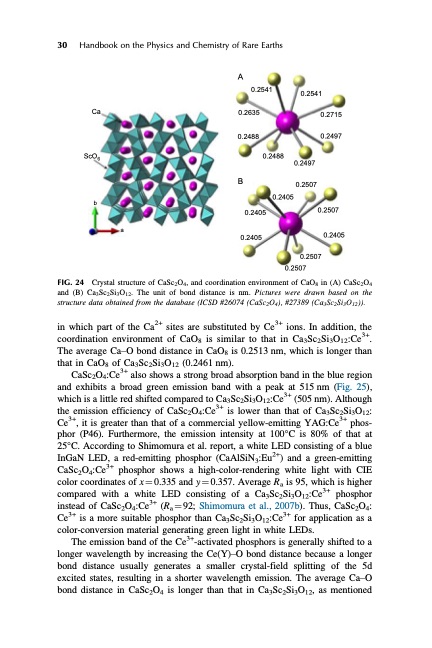
30 Handbook on the Physics and Chemistry of Rare Earths A FIG. 24 Ca ScO6 b 0.2541 0.2635 0.2488 B 0.2405 0.2405 0.2541 0.2715 0.2497 0.2507 0.2405 a Crystal structure of CaSc2O4, and coordination environment of CaO8 in (A) CaSc2O4 and (B) Ca3Sc2Si3O12. The unit of bond distance is nm. Pictures were drawn based on the structure data obtained from the database (ICSD #26074 (CaSc2O4), #27389 (Ca3Sc2Si3O12)). in which part of the Ca2+ sites are substituted by Ce3+ ions. In addition, the coordination environment of CaO8 is similar to that in Ca3Sc2Si3O12:Ce3+. The average Ca–O bond distance in CaO8 is 0.2513 nm, which is longer than that in CaO8 of Ca3Sc2Si3O12 (0.2461 nm). CaSc2O4:Ce3+ also shows a strong broad absorption band in the blue region and exhibits a broad green emission band with a peak at 515 nm (Fig. 25), which is a little red shifted compared to Ca3Sc2Si3O12:Ce3+ (505 nm). Although the emission efficiency of CaSc2O4:Ce3+ is lower than that of Ca3Sc2Si3O12: Ce3+, it is greater than that of a commercial yellow-emitting YAG:Ce3+ phos- phor (P46). Furthermore, the emission intensity at 100°C is 80% of that at 25°C. According to Shimomura et al. report, a white LED consisting of a blue InGaN LED, a red-emitting phosphor (CaAlSiN3:Eu2+) and a green-emitting CaSc2O4:Ce3+ phosphor shows a high-color-rendering white light with CIE color coordinates of x1⁄40.335 and y1⁄40.357. Average Ra is 95, which is higher compared with a white LED consisting of a Ca3Sc2Si3O12:Ce3+ phosphor instead of CaSc2O4:Ce3+ (Ra1⁄492; Shimomura et al., 2007b). Thus, CaSc2O4: Ce3+ is a more suitable phosphor than Ca3Sc2Si3O12:Ce3+ for application as a color-conversion material generating green light in white LEDs. The emission band of the Ce3+-activated phosphors is generally shifted to a longer wavelength by increasing the Ce(Y)–O bond distance because a longer bond distance usually generates a smaller crystal-field splitting of the 5d excited states, resulting in a shorter wavelength emission. The average Ca–O bond distance in CaSc2O4 is longer than that in Ca3Sc2Si3O12, as mentioned 0.2488 0.2497 0.2507 0.2405 0.2507 0.2507PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |