
PDF Publication Title:
Text from PDF Page: 105
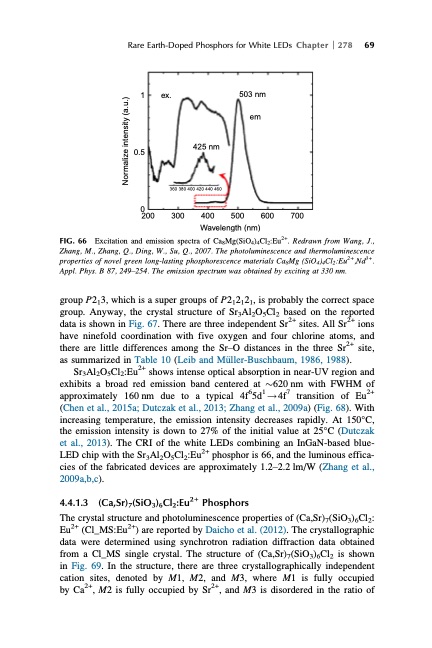
FIG. 66 Rare Earth-Doped Phosphors for White LEDs Chapter 278 69 1 0.5 ex. 503 nm em 425 nm 360 380 400 420 440 460 0 200 300 400 Excitation and emission spectra of Ca8Mg(SiO4)4Cl2:Eu2+. Redrawn from Wang, J., Zhang, M., Zhang, Q., Ding, W., Su, Q., 2007. The photoluminescence and thermoluminescence properties of novel green long-lasting phosphorescence materials Ca8Mg (SiO4)4Cl2:Eu2+,Nd3+. Appl. Phys. B 87, 249–254. The emission spectrum was obtained by exciting at 330 nm. group P213, which is a super groups of P212121, is probably the correct space group. Anyway, the crystal structure of Sr3Al2O5Cl2 based on the reported data is shown in Fig. 67. There are three independent Sr2+ sites. All Sr2+ ions have ninefold coordination with five oxygen and four chlorine atoms, and there are little differences among the Sr–O distances in the three Sr2+ site, as summarized in Table 10 (Leib and M€uller-Buschbaum, 1986, 1988). Sr3Al2O5Cl2:Eu2+ shows intense optical absorption in near-UV region and exhibits a broad red emission band centered at $620 nm with FWHM of approximately 160 nm due to a typical 4f65d1 ! 4f7 transition of Eu2+ (Chen et al., 2015a; Dutczak et al., 2013; Zhang et al., 2009a) (Fig. 68). With increasing temperature, the emission intensity decreases rapidly. At 150°C, the emission intensity is down to 27% of the initial value at 25°C (Dutczak et al., 2013). The CRI of the white LEDs combining an InGaN-based blue- LED chip with the Sr3Al2O5Cl2:Eu2+ phosphor is 66, and the luminous effica- cies of the fabricated devices are approximately 1.2–2.2 lm/W (Zhang et al., 2009a,b,c). 4.4.1.3 (Ca,Sr)7(SiO3)6Cl2:Eu2+ Phosphors The crystal structure and photoluminescence properties of (Ca,Sr)7(SiO3)6Cl2: Eu2+ (Cl_MS:Eu2+) are reported by Daicho et al. (2012). The crystallographic data were determined using synchrotron radiation diffraction data obtained from a Cl_MS single crystal. The structure of (Ca,Sr)7(SiO3)6Cl2 is shown in Fig. 69. In the structure, there are three crystallographically independent cation sites, denoted by M1, M2, and M3, where M1 is fully occupied by Ca2+, M2 is fully occupied by Sr2+, and M3 is disordered in the ratio of 500 600 700 Wavelength (nm) Normalize intensity (a.u.)PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |