
PDF Publication Title:
Text from PDF Page: 108
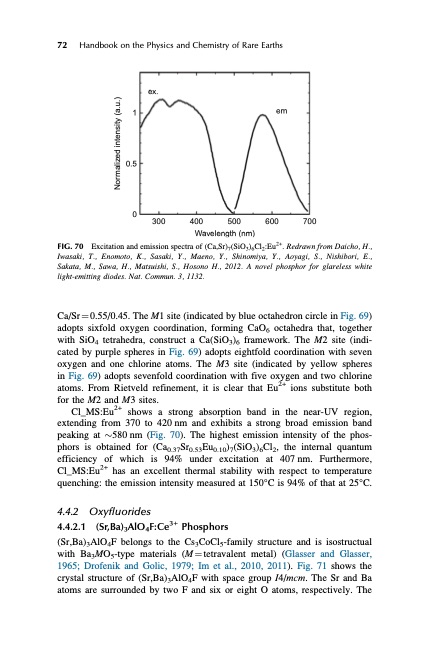
72 Handbook on the Physics and Chemistry of Rare Earths ex. 1 0.5 0 em 300 400 Wavelength (nm) Excitation and emission spectra of (Ca,Sr)7(SiO3)6Cl2:Eu2+. Redrawn from Daicho, H., Iwasaki, T., Enomoto, K., Sasaki, Y., Maeno, Y., Shinomiya, Y., Aoyagi, S., Nishibori, E., Sakata, M., Sawa, H., Matsuishi, S., Hosono H., 2012. A novel phosphor for glareless white light-emitting diodes. Nat. Commun. 3, 1132. Ca/Sr 1⁄4 0.55/0.45. The M1 site (indicated by blue octahedron circle in Fig. 69) adopts sixfold oxygen coordination, forming CaO6 octahedra that, together with SiO4 tetrahedra, construct a Ca(SiO3)6 framework. The M2 site (indi- cated by purple spheres in Fig. 69) adopts eightfold coordination with seven oxygen and one chlorine atoms. The M3 site (indicated by yellow spheres in Fig. 69) adopts sevenfold coordination with five oxygen and two chlorine atoms. From Rietveld refinement, it is clear that Eu2+ ions substitute both for the M2 and M3 sites. Cl_MS:Eu2+ shows a strong absorption band in the near-UV region, extending from 370 to 420 nm and exhibits a strong broad emission band peaking at $580 nm (Fig. 70). The highest emission intensity of the phos- phors is obtained for (Ca0.37Sr0.53Eu0.10)7(SiO3)6Cl2, the internal quantum efficiency of which is 94% under excitation at 407nm. Furthermore, Cl_MS:Eu2+ has an excellent thermal stability with respect to temperature quenching: the emission intensity measured at 150°C is 94% of that at 25°C. 4.4.2 Oxyfluorides 4.4.2.1 (Sr,Ba)3AlO4F:Ce3+ Phosphors (Sr,Ba)3AlO4F belongs to the Cs3CoCl5-family structure and is isostructual with Ba3MO5-type materials (M1⁄4tetravalent metal) (Glasser and Glasser, 1965; Drofenik and Golic, 1979; Im et al., 2010, 2011). Fig. 71 shows the crystal structure of (Sr,Ba)3AlO4F with space group I4/mcm. The Sr and Ba atoms are surrounded by two F and six or eight O atoms, respectively. The FIG. 70 500 600 700 Normalized intensity (a.u.)PDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |