
PDF Publication Title:
Text from PDF Page: 014
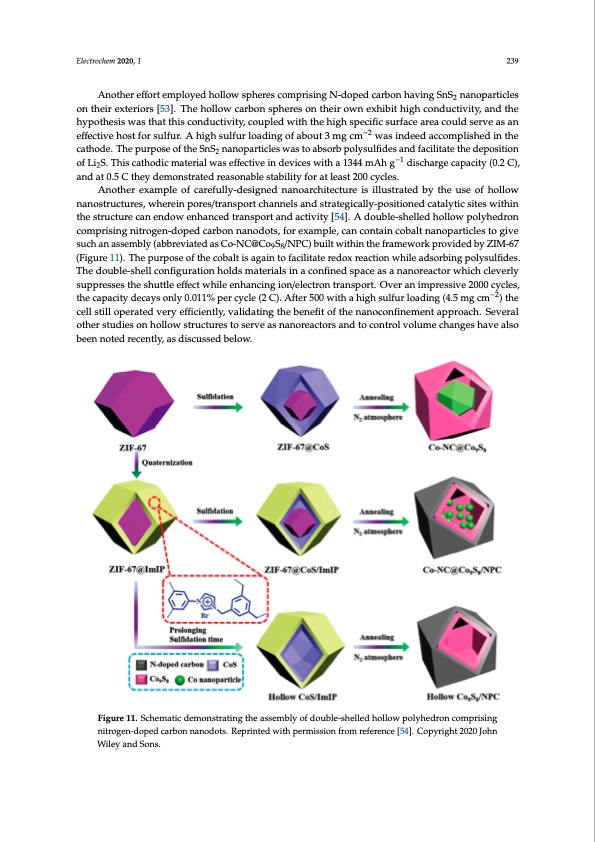
Electrochem 2020, 1 239 Another effort employed hollow spheres comprising N-doped carbon having SnS2 nanoparticles on their exteriors [53]. The hollow carbon spheres on their own exhibit high conductivity, and the hypothesis was that this conductivity, coupled with the high specific surface area could serve as an effective host for sulfur. A high sulfur loading of about 3 mg cm−2 was indeed accomplished in the cathode. The purpose of the SnS2 nanoparticles was to absorb polysulfides and facilitate the deposition of Li2S. This cathodic material was effective in devices with a 1344 mAh g−1 discharge capacity (0.2 C), and at 0.5 C they demonstrated reasonable stability for at least 200 cycles. Another example of carefully-designed nanoarchitecture is illustrated by the use of hollow nanostructures, wherein pores/transport channels and strategically-positioned catalytic sites within Electrochem 2020, 2, FOR PEER REVIEW 15 the structure can endow enhanced transport and activity [54]. A double-shelled hollow polyhedron comprising nitrogen-doped carbon nanodots, for example, can contain cobalt nanoparticles to give comprising nitrogen-doped carbon nanodots, for example, can contain cobalt nanoparticles to give such an assembly (abbreviated as Co-NC@Co S /NPC) built within the framework provided by ZIM-67 98 such an assembly (abbreviated as Co-NC@Co9S8/NPC) built within the framework provided by ZIM- (Figure 11). The purpose of the cobalt is again to facilitate redox reaction while adsorbing polysulfides. 67 (Figure 11). The purpose of the cobalt is again to facilitate redox reaction while adsorbing The double-shell configuration holds materials in a confined space as a nanoreactor which cleverly polysulfides. The double-shell configuration holds materials in a confined space as a nanoreactor suppresses the shuttle effect while enhancing ion/electron transport. Over an impressive 2000 cycles, which cleverly suppresses the shuttle effect while enhancing ion/electron transport. Over an the capacity decays only 0.011% per cycle (2 C). After 500 with a high sulfur loading (4.5 mg cm−2) the impressive 2000 cycles, the capacity decays only 0.011% per cycle (2 C). After 500 with a high sulfur cell still operated very−2efficiently, validating the benefit of the nanoconfinement approach. Several loading (4.5 mg cm ) the cell still operated very efficiently, validating the benefit of the other studies on hollow structures to serve as nanoreactors and to control volume changes have also nanoconfinement approach. Several other studies on hollow structures to serve as nanoreactors and been noted recently, as discussed below. to control volume changes have also been noted recently, as discussed below. Figure 11. Schematic demonstrating the assembly of double-shelled hollow polyhedron comprising Figure 11. Schematic demonstrating the assembly of double-shelled hollow polyhedron comprising nitrogen-doped carbon nanodots. Reprinted with permission from reference [54]. Copyright 2020 John nitrogen-doped carbon nanodots. Reprinted with permission from reference [54]. Copyright 2020 Wiley and Sons. John Wiley and Sons. Despite the benefits afforded by hollow pore-containing systems, there is some drawback to having such a high sulfur loading due to a lack of ability for all the sulfur to interact with electrolyte. In a recent study, researchers have addressed this by installing rib-like supports within their hollow carbon structure (Figure 12) [55]. Resultant radially-aligned systems have a very well-defined set of pores, facilitating the efficient infusion of sulfur into the channels. To further enhance the propensity of this system to block polysulfide species, nitrogen-doped carbon nanospheres were also employedPDF Image | Lithium-Sulfur Batteries: Advances and Trends
PDF Search Title:
Lithium-Sulfur Batteries: Advances and TrendsOriginal File Name Searched:
electrochem-01-00016.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |