
PDF Publication Title:
Text from PDF Page: 019
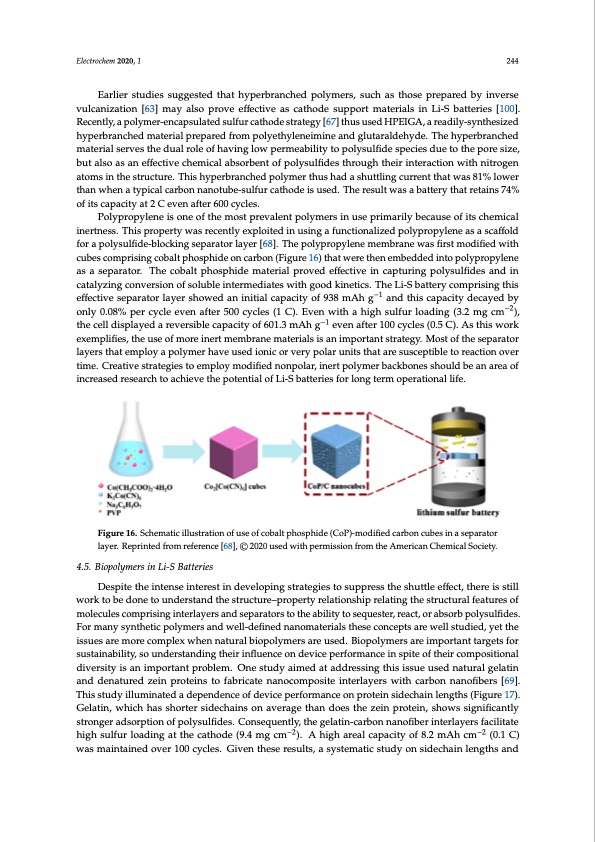
Electrochem 2020, 1 244 Earlier studies suggested that hyperbranched polymers, such as those prepared by inverse vulcanization [63] may also prove effective as cathode support materials in Li-S batteries [100]. Recently, a polymer-encapsulated sulfur cathode strategy [67] thus used HPEIGA, a readily-synthesized hyperbranched material prepared from polyethyleneimine and glutaraldehyde. The hyperbranched material serves the dual role of having low permeability to polysulfide species due to the pore size, but also as an effective chemical absorbent of polysulfides through their interaction with nitrogen Electrochem 2020, 2, FOR PEER REVIEW 20 atoms in the structure. This hyperbranched polymer thus had a shuttling current that was 81% lower thwanasw8h1%enloawtyepritchaalncawrhbeonantaynpoitcuabl cea-srubolfnurnacantohtuodbe-issuulfsuerdc.aTthhoedreeisuultsewda.sTahebraetsteurlyt wthaastarebtatitnersy74% ofthitastcraeptaicnisty74a%t 2oCf itesvceanpacftietyr a6t020 Ccyecvlesn. after 600 cycles. Polypropylene is one of the most prevalent polymers in use primarily because of its chemical Polypropylene is one of the most prevalent polymers in use primarily because of its chemical inertness. This property was recently exploited in using a functionalized polypropylene as a scaffold inertness. This property was recently exploited in using a functionalized polypropylene as a scaffold for a polysulfide-blocking separator layer [68]. The polypropylene membrane was first modified with for a polysulfide-blocking separator layer [68]. The polypropylene membrane was first modified with cubes comprising cobalt phosphide on carbon (Figure 16) that were then embedded into cubes comprising cobalt phosphide on carbon (Figure 16) that were then embedded into polypropylene polypropylene as a separator. The cobalt phosphide material proved effective in capturing as a separator. The cobalt phosphide material proved effective in capturing polysulfides and in polysulfides and in catalyzing conversion of soluble intermediates with good kinetics. The Li-S catalyzing conversion of soluble intermediates with good kinetics. The Li-S battery comprising this battery comprising this effective separator layer showed an initial capacity of 938 mAh g−1 and this effective separator layer showed an initial capacity of 938 mAh g−1 and this capacity decayed by capacity decayed by only 0.08% per cycle even after 500 cycles (1 C). Even with a high sulfur loading −2 only 0.08% per cycle even after 500 cycles (1 C). Even with a high sulfur loading (3.2 mg cm ), (3.2 mg cm−2), the cell displayed a reversible capacity of 601.3 mAh g−1 even after 100 cycles (0.5 C). the cell displayed a reversible capacity of 601.3 mAh g−1 even after 100 cycles (0.5 C). As this work As this work exemplifies, the use of more inert membrane materials is an important strategy. Most exemplifies, the use of more inert membrane materials is an important strategy. Most of the separator of the separator layers that employ a polymer have used ionic or very polar units that are susceptible layers that employ a polymer have used ionic or very polar units that are susceptible to reaction over to reaction over time. Creative strategies to employ modified nonpolar, inert polymer backbones time. Creative strategies to employ modified nonpolar, inert polymer backbones should be an area of should be an area of increased research to achieve the potential of Li-S batteries for long term increased research to achieve the potential of Li-S batteries for long term operational life. operational life. FiFgiugurere161.6S.cShcehmemataictiiclluilslutrsattriaotnioonfuofseuosfecobfaclotbpahlotsphoidspeh(iCdoeP()C-moPo)d-imfioedicfiaerdbocnarcbuobnesciunbaeseipnaraator lasyeepra.rRaetoprilnatyeedr.frRoemprienfteerdenfcreom[68r]e,f©ere2n0c2e0u[6s8e]d, ©wi2th02p0erumseisdsiwonithfropmertmhiessAiomnerfricoamnCtheemAmicaelriScoacniety. Chemical Society. 4.5. Biopolymers in Li-S Batteries 4.5. Biopolymers in Li-S Batteries Despite the intense interest in developing strategies to suppress the shuttle effect, there is still work tDoebsepidteontheetoinutendserisnttaenrdesthien sdteruvecltouprein–gprsotrpaetertgyiersetlaotsiounpsphriepssrethlaetisnhgutthle esftfreucctt,uthraelrefeiastsutrilels of mwoloerckutleosbceodmopnreistionugnindteerrsltaynedrsthaensdtrsuecptauraet–oprrsotpoetrhtyeareblialittiyontoshsiepqrueelastienr,grtehaects,torurcatbusroarlbfepaotulyresuslofifdes. Fomromleacnuylesyncothmeptircispinoglyminetersrlaynedrswealnl-desfienpeadrantoarnsomtoatehreiaalsbtilhiteysetcoonsecqeputestaere, wrealcl ts,tuodrieadb,syoerbt the ispsuoelyssaurlefidmeos.reFocrompanleyxswynhtehnetnicaptuorlaylmbeirospaonlydmweerlsl-adreefiuneseddn.aBniompoatleyrmiaelsrsthaerseeimcopnocertpatnstatraerwgetlsl for studied, yet the issues are more complex when natural biopolymers are used. Biopolymers are sustainability, so understanding their influence on device performance in spite of their compositional important targets for sustainability, so understanding their influence on device performance in spite diversity is an important problem. One study aimed at addressing this issue used natural gelatin of their compositional diversity is an important problem. One study aimed at addressing this issue and denatured zein proteins to fabricate nanocomposite interlayers with carbon nanofibers [69]. used natural gelatin and denatured zein proteins to fabricate nanocomposite interlayers with carbon This study illuminated a dependence of device performance on protein sidechain lengths (Figure 17). nanofibers [69]. This study illuminated a dependence of device performance on protein sidechain Gelatin, which has shorter sidechains on average than does the zein protein, shows significantly lengths (Figure 17). Gelatin, which has shorter sidechains on average than does the zein protein, stronger adsorption of polysulfides. Consequently, the gelatin-carbon nanofiber interlayers facilitate shows significantly stronger adsorption of polysulfides. Consequently, the gelatin-carbon nanofiber high sulfur loading at the cathode (9.4 mg cm−2). A high areal capacity of 8.2 mAh cm−2 (0.1 C) interlayers facilitate high sulfur loading at the cathode (9.4 mg cm−2). A high areal capacity of 8.2 mAh was maintained over 100 cycles. Given these results, a systematic study on sidechain lengths and cm−2 (0.1 C) was maintained over 100 cycles. Given these results, a systematic study on sidechain lengths and composition employing polar proteins like gelatin could be an important next step to fully delineating the structure–performance understanding.PDF Image | Lithium-Sulfur Batteries: Advances and Trends
PDF Search Title:
Lithium-Sulfur Batteries: Advances and TrendsOriginal File Name Searched:
electrochem-01-00016.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |