
PDF Publication Title:
Text from PDF Page: 021
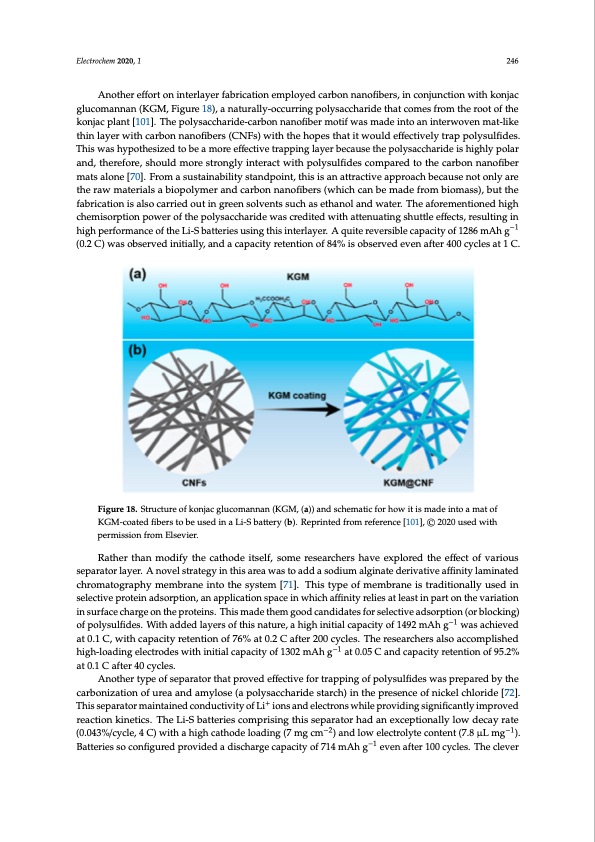
Electrochem 2020, 1 246 Another effort on interlayer fabrication employed carbon nanofibers, in conjunction with konjac gEleuctcrocmheamn2n0a2n0, 2(K, FGOMR P,EFEiRguRrEeV1IE8W), a naturally-occurring polysaccharide that comes from the root of th2e2 konjac plant [101]. The polysaccharide-carbon nanofiber motif was made into an interwoven mat-like This was hypothesized to be a more effective trapping layer because the polysaccharide is highly thin layer with carbon nanofibers (CNFs) with the hopes that it would effectively trap polysulfides. polar and, therefore, should more strongly interact with polysulfides compared to the carbon This was hypothesized to be a more effective trapping layer because the polysaccharide is highly polar nanofiber mats alone [70]. From a sustainability standpoint, this is an attractive approach because not and, therefore, should more strongly interact with polysulfides compared to the carbon nanofiber only are the raw materials a biopolymer and carbon nanofibers (which can be made from biomass), mats alone [70]. From a sustainability standpoint, this is an attractive approach because not only are but the fabrication is also carried out in green solvents such as ethanol and water. The aforementioned the raw materials a biopolymer and carbon nanofibers (which can be made from biomass), but the high chemisorption power of the polysaccharide was credited with attenuating shuttle effects, fabrication is also carried out in green solvents such as ethanol and water. The aforementioned high resulting in high performance of the Li-S batteries using this interlayer. A quite reversible capacity of chemisorption power of the polysaccharide was credited with attenuating shuttle effects, resulting in 1286 mAh g−1 (0.2 C) was observed initially, and a capacity retention of 84% is observed even aft−e1r high performance of the Li-S batteries using this interlayer. A quite reversible capacity of 1286 mAh g 400 cycles at 1 C. (0.2 C) was observed initially, and a capacity retention of 84% is observed even after 400 cycles at 1 C. Figure 18. Structure of konjac glucomannan (KGM, a) and schematic for how it is made into a mat of Figure 18. Structure of konjac glucomannan (KGM, (a)) and schematic for how it is made into a mat of KGM-coated fibers to be used in a Li-S battery (b). Reprinted from reference [101], © 2020 used with KGM-coated fibers to be used in a Li-S battery (b). Reprinted from reference [101], © 2020 used with permission from Elsevier. permission from Elsevier. Rather than modify the cathode itself, some researchers have explored the effffect of various separattorlalayyere.r.AAnonvoevlsetlrastergatyeignythinisathreisawaraesatowaadsdtaosaoddiuma asolgdiniuamtedaelgrivnattievedaeffirivnaittyivlaemaifnfiantietdy clahmroimnatteodgrcahprohmy amtoegmrabprahnyeminetmobtrhaenseyisntteomth[e71s]y.stTehmis[t7y1p].eTohfismtyempeborafnmeeims tbrraadnietioisntarlalydiutisoendalilny suesledctivnesperloectetivneadpsrortepitnioand, saonrapptipolnic,aatnionapsplaiceatiinonwhspicahceaffiinnwityhricehlieasffaitnlietyasrteinliepsaart olenatshteinvapraiartion itnhesuvrfarcieatcihoanrgineosnutrhfaecperoctheainrsg.eTohnismthaedeprthoetemingso.oTdhcisanmdiaddaetesthfoemrsegleocotidvecadnsdoidrpatieosnf(orbsleolcekcitnivge) −1 oafdpsorlypstuiolnfid(eosr.bWloicthkiandgd)eodf lpaoyleyrsuolfitdheis.nWatiuthre,aaddheigdhlainyietiraslocfapthaicsitnyaotfu1re4,9a2 mhiAgh ignitiawlacsaapcahcietyveodf −1 a1t4902.1mCA, whithg cawpasciatychrietveendtioant 0o.f17C6%, waitth0.2caCpacfitteyr 2re0t0encyticolneso. fT7h6e%reastea0r.2chCerasfatelsro2a0c0ccoymclpelsi.shTehde −1 −1 hreigseha-lrocahdeirnsgaelsloecatrcocdoemspwlistheidnihtiiaglhc-alpoacdiitnygoefl1e3c0tr2omdeAshwgithainti0t.i0a5l cCapanacditcyapoafc1i3ty02remteAntihongofa9t50.2.0%5 aCt a0n.1dCcapftaecrit4y0 rceytcelnetsi.on of 95.2% at 0.1 C after 40 cycles. Another type of separator that proved effffective for trapping of polysulfifides was prepared by the carbonization of urea and amylose (a polysaccharide starch) in the presence of nickel chloride [72]. ++ Thiissespeparaartaotromr aminatianitnaeindecdoncdouncdtiuvcityivoitfyLiofioLnis ainodnseleacntdroneslewcthriolenps rwovhidiliengpsriogvniidfiicnagntslyiginmifpicraonvteldy rimeapctriovnedkinreaticctsio.nThkeinLeit-iScsb.aTthteriLeis-Scobmatpterrisieinsgcothmispsriespinagrathorishsaedparnateoxrcehpatdioannalelyxcleopwtiodneacallyrlaotwe −2−2 −1 (d0e.0c4a3y%r/actyec(le0,.044C3%) w/ciythclea,h4igCh)cwatihthodaehloigahdicnagth(7odmeglocamdin)ga(n7dmlogwcmelec)traonlydtelocwonetleenctr(o7.l8ytμeLcomngten)t. −1 −1 −1 B(7a.t8teμriLesmsog c)o.nBfiagtuterreidespsrovciodnefdigaudreisdchparrogveidcaepdaacidtyisocfh7a1r4gemcAaphagcityeovfen71a4ftmerA10h0 cgyclevse. nThaefteclre1v0e0r cycles. The clever design of this system provides insight into future systems wherein catalytic nanoparticles can be combined with more traditional layer architectures. As the synopses of the last few studies reveal, polar biopolymers like gelatin and polysaccharides have good barrier properties for suppressing the shuttle effect. The variable and complex composition of biopolymers have been cited as a drawback of trying to use them on a large scale, however, leading some researchers to evaluate highly polar synthetic polymers. In one suchPDF Image | Lithium-Sulfur Batteries: Advances and Trends
PDF Search Title:
Lithium-Sulfur Batteries: Advances and TrendsOriginal File Name Searched:
electrochem-01-00016.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |