
PDF Publication Title:
Text from PDF Page: 026
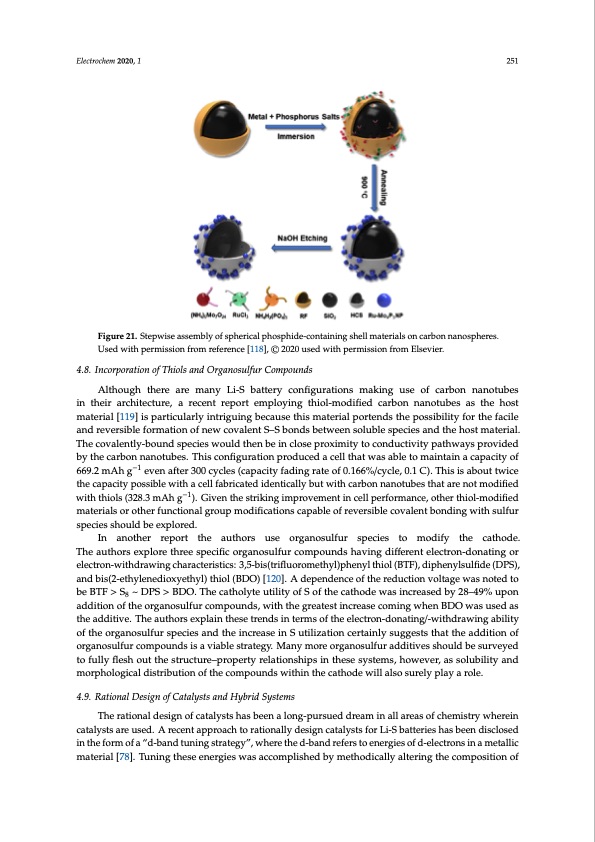
Electrochem 2020, 1 251 Electrochem 2020, 2, FOR PEER REVIEW 27 Figure 21. Stepwise assembly of spherical phosphide-containing shell materials on carbon nanospheres. Figure 21. Stepwise assembly of spherical phosphide-containing shell materials on carbon Used with permission from reference [118], © 2020 used with permission from Elsevier. nanospheres. Used with permission from reference [118], © 2020 used with permission from Elsevier. 4.8. Incorporation of Thiols and Organosulfur Compounds 4.8. Incorporation of Thiols and Organosulfur Compounds Although there are many Li-S battery configurations making use of carbon nanotubes Although there are many Li-S battery configurations making use of carbon nanotubes in their in their architecture, a recent report employing thiol-modified carbon nanotubes as the host architecture, a recent report employing thiol-modified carbon nanotubes as the host material [119] is material [119] is particularly intriguing because this material portends the possibility for the facile particularly intriguing because this material portends the possibility for the facile and reversible and reversible formation of new covalent S–S bonds between soluble species and the host material. formation of new covalent S–S bonds between soluble species and the host material. The covalently- The covalently-bound species would then be in close proximity to conductivity pathways provided bound species would then be in close proximity to conductivity pathways provided by the carbon by the carbon nanotubes. This configuration produced a cell that was able to maintain a capacity of nanotubes. This configuration produced a cell that was able to maintain a capacity of 669.2 mAh g−1 669.2 mAh g−1 even after 300 cycles (capacity fading rate of 0.166%/cycle, 0.1 C). This is about twice even after 300 cycles (capacity fading rate of 0.166%/cycle, 0.1 C). This is about twice the capacity the capacity possible with a cell fabricated identically but with carbon nanotubes that are not modified possible with a cell fabricated identically but with carbon nanotubes that are not modified with thiols with thiols (328.3 mAh g−1). Given the striking improvement in cell performance, other thiol-modified (328.3 mAh g−1). Given the striking improvement in cell performance, other thiol-modified materials materials or other functional group modifications capable of reversible covalent bonding with sulfur or other functional group modifications capable of reversible covalent bonding with sulfur species species should be explored. should be explored. In another report the authors use organosulfur species to modify the cathode. In another report the authors use organosulfur species to modify the cathode. The authors The authors explore three specific organosulfur compounds having different electron-donating or explore three specific organosulfur compounds having different electron-donating or electron- electron-withdrawing characteristics: 3,5-bis(trifluoromethyl)phenyl thiol (BTF), diphenylsulfide (DPS), withdrawing characteristics: 3,5-bis(trifluoromethyl)phenyl thiol (BTF), diphenylsulfide (DPS), and and bis(2-ethylenedioxyethyl) thiol (BDO) [120]. A dependence of the reduction voltage was noted to bis(2-ethylenedioxyethyl) thiol (BDO) [120]. A dependence of the reduction voltage was noted to be be BTF > S8 ~ DPS > BDO. The catholyte utility of S of the cathode was increased by 28–49% upon BTF > S8 ~ DPS > BDO. The catholyte utility of S of the cathode was increased by 28–49% upon addition of the organosulfur compounds, with the greatest increase coming when BDO was used as addition of the organosulfur compounds, with the greatest increase coming when BDO was used as the additive. The authors explain these trends in terms of the electron-donating/-withdrawing ability the additive. The authors explain these trends in terms of the electron-donating/-withdrawing ability of the organosulfur species and the increase in S utilization certainly suggests that the addition of of the organosulfur species and the increase in S utilization certainly suggests that the addition of organosulfur compounds is a viable strategy. Many more organosulfur additives should be surveyed organosulfur compounds is a viable strategy. Many more organosulfur additives should be surveyed to fully flesh out the structure–property relationships in these systems, however, as solubility and to fully flesh out the structure–property relationships in these systems, however, as solubility and morphological distribution of the compounds within the cathode will also surely play a role. morphological distribution of the compounds within the cathode will also surely play a role. 4.9. Rational Design of Catalysts and Hybrid Systems 4.9. Rational Design of Catalysts and Hybrid Systems The rational design of catalysts has been a long-pursued dream in all areas of chemistry wherein The rational design of catalysts has been a long-pursued dream in all areas of chemistry wherein catalysts are used. A recent approach to rationally design catalysts for Li-S batteries has been disclosed catalysts are used. A recent approach to rationally design catalysts for Li-S batteries has been in the form of a “d-band tuning strategy”, where the d-band refers to energies of d-electrons in a metallic disclosed in the form of a “d-band tuning strategy”, where the d-band refers to energies of d-electrons material [78]. Tuning these energies was accomplished by methodically altering the composition of in a metallic material [78]. Tuning these energies was accomplished by methodically altering the composition of cobalt alloys with nickel phosphide (Ni2P). Addition of the Ni2P increases interaction with polysulfides and in this study additional activity of species identified as Ni2Co4P3 wasPDF Image | Lithium-Sulfur Batteries: Advances and Trends
PDF Search Title:
Lithium-Sulfur Batteries: Advances and TrendsOriginal File Name Searched:
electrochem-01-00016.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |