
PDF Publication Title:
Text from PDF Page: 018
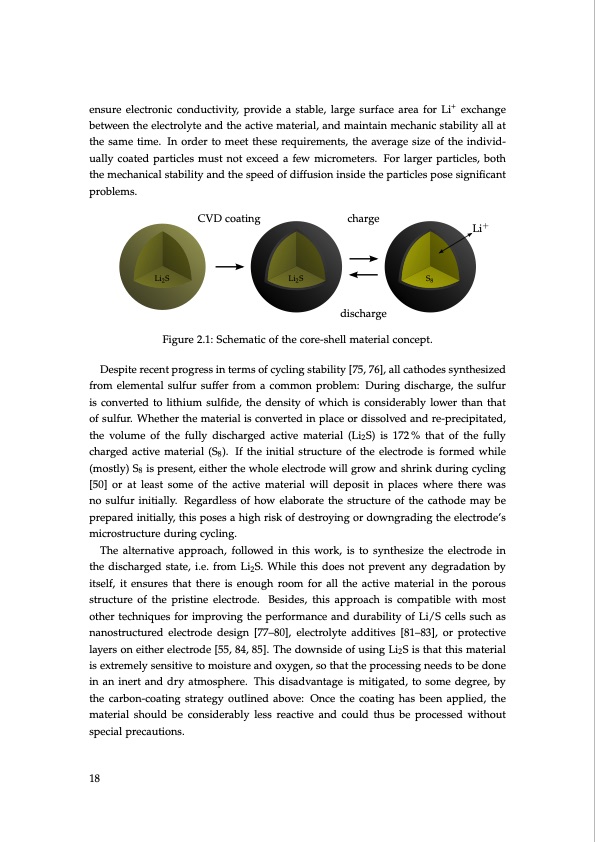
ensure electronic conductivity, provide a stable, large surface area for Li+ exchange between the electrolyte and the active material, and maintain mechanic stability all at the same time. In order to meet these requirements, the average size of the individ- ually coated particles must not exceed a few micrometers. For larger particles, both the mechanical stability and the speed of diffusion inside the particles pose significant problems. CVD coating charge discharge Li+ Li2S Li2S S8 Figure 2.1: Schematic of the core-shell material concept. Despite recent progress in terms of cycling stability [75, 76], all cathodes synthesized from elemental sulfur suffer from a common problem: During discharge, the sulfur is converted to lithium sulfide, the density of which is considerably lower than that of sulfur. Whether the material is converted in place or dissolved and re-precipitated, the volume of the fully discharged active material (Li2S) is 172% that of the fully charged active material (S8). If the initial structure of the electrode is formed while (mostly) S8 is present, either the whole electrode will grow and shrink during cycling [50] or at least some of the active material will deposit in places where there was no sulfur initially. Regardless of how elaborate the structure of the cathode may be prepared initially, this poses a high risk of destroying or downgrading the electrode’s microstructure during cycling. The alternative approach, followed in this work, is to synthesize the electrode in the discharged state, i.e. from Li2S. While this does not prevent any degradation by itself, it ensures that there is enough room for all the active material in the porous structure of the pristine electrode. Besides, this approach is compatible with most other techniques for improving the performance and durability of Li/S cells such as nanostructured electrode design [77–80], electrolyte additives [81–83], or protective layers on either electrode [55, 84, 85]. The downside of using Li2S is that this material is extremely sensitive to moisture and oxygen, so that the processing needs to be done in an inert and dry atmosphere. This disadvantage is mitigated, to some degree, by the carbon-coating strategy outlined above: Once the coating has been applied, the material should be considerably less reactive and could thus be processed without special precautions. 18PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |