
PDF Publication Title:
Text from PDF Page: 026
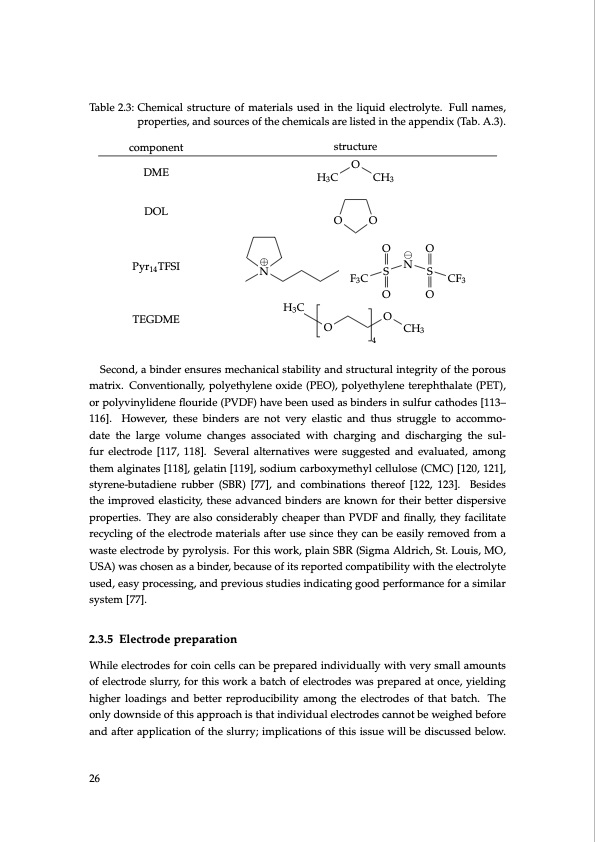
Table 2.3: Chemical structure of materials used in the liquid electrolyte. Full names, properties, and sources of the chemicals are listed in the appendix (Tab. A.3). component DME DOL Pyr14 TFSI TEGDME structure O CH3 OO O⊖O ⊕N N F3CSSCF3 OO H3C H3C O O 4 CH3 Second, a binder ensures mechanical stability and structural integrity of the porous matrix. Conventionally, polyethylene oxide (PEO), polyethylene terephthalate (PET), or polyvinylidene flouride (PVDF) have been used as binders in sulfur cathodes [113– 116]. However, these binders are not very elastic and thus struggle to accommo- date the large volume changes associated with charging and discharging the sul- fur electrode [117, 118]. Several alternatives were suggested and evaluated, among them alginates [118], gelatin [119], sodium carboxymethyl cellulose (CMC) [120, 121], styrene-butadiene rubber (SBR) [77], and combinations thereof [122, 123]. Besides the improved elasticity, these advanced binders are known for their better dispersive properties. They are also considerably cheaper than PVDF and finally, they facilitate recycling of the electrode materials after use since they can be easily removed from a waste electrode by pyrolysis. For this work, plain SBR (Sigma Aldrich, St. Louis, MO, USA) was chosen as a binder, because of its reported compatibility with the electrolyte used, easy processing, and previous studies indicating good performance for a similar system [77]. 2.3.5 Electrode preparation While electrodes for coin cells can be prepared individually with very small amounts of electrode slurry, for this work a batch of electrodes was prepared at once, yielding higher loadings and better reproducibility among the electrodes of that batch. The only downside of this approach is that individual electrodes cannot be weighed before and after application of the slurry; implications of this issue will be discussed below. 26PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |