
PDF Publication Title:
Text from PDF Page: 045
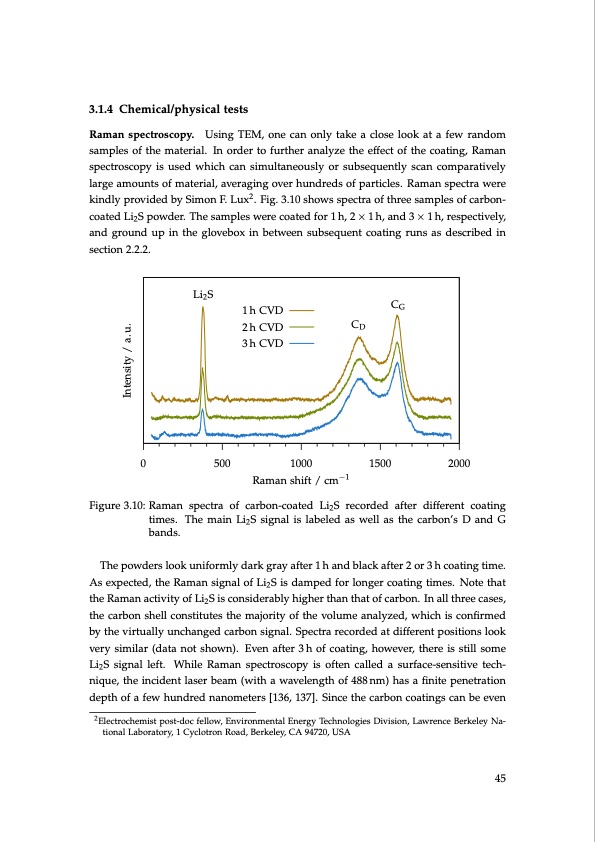
3.1.4 Chemical/physical tests Raman spectroscopy. Using TEM, one can only take a close look at a few random samples of the material. In order to further analyze the effect of the coating, Raman spectroscopy is used which can simultaneously or subsequently scan comparatively large amounts of material, averaging over hundreds of particles. Raman spectra were kindly provided by Simon F. Lux2. Fig. 3.10 shows spectra of three samples of carbon- coated Li2S powder. The samples were coated for 1 h, 2 × 1 h, and 3 × 1 h, respectively, and ground up in the glovebox in between subsequent coating runs as described in section 2.2.2. Li2 S 1 h CVD 2 h CVD 3 h CVD 500 1000 Raman shift / cm−1 CG CD Intensity / a. u. 0 1500 2000 Figure 3.10: Raman spectra of carbon-coated Li2S recorded after different coating times. The main Li2S signal is labeled as well as the carbon’s D and G bands. The powders look uniformly dark gray after 1 h and black after 2 or 3 h coating time. As expected, the Raman signal of Li2S is damped for longer coating times. Note that the Raman activity of Li2S is considerably higher than that of carbon. In all three cases, the carbon shell constitutes the majority of the volume analyzed, which is confirmed by the virtually unchanged carbon signal. Spectra recorded at different positions look very similar (data not shown). Even after 3 h of coating, however, there is still some Li2S signal left. While Raman spectroscopy is often called a surface-sensitive tech- nique, the incident laser beam (with a wavelength of 488 nm) has a finite penetration depth of a few hundred nanometers [136, 137]. Since the carbon coatings can be even 2Electrochemist post-doc fellow, Environmental Energy Technologies Division, Lawrence Berkeley Na- tional Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA 45PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |