
PDF Publication Title:
Text from PDF Page: 054
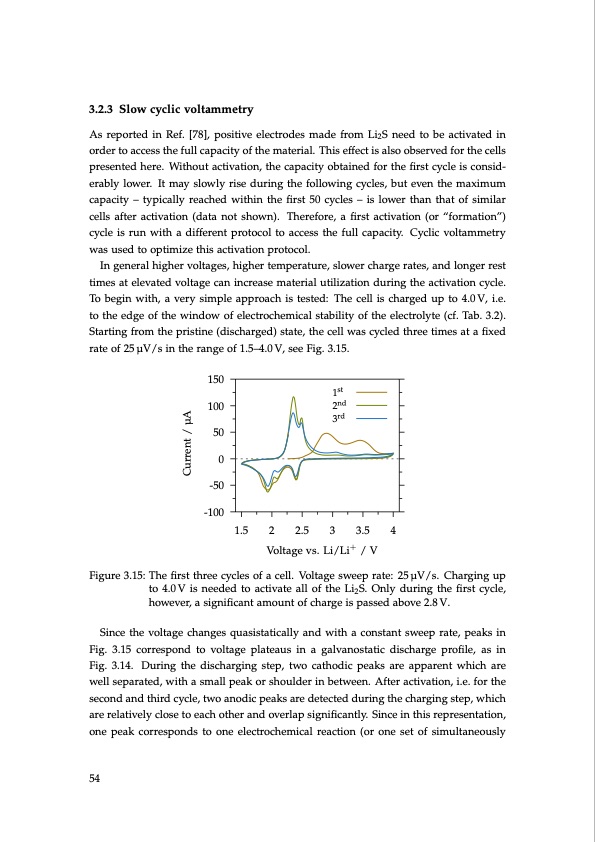
3.2.3 Slow cyclic voltammetry As reported in Ref. [78], positive electrodes made from Li2S need to be activated in order to access the full capacity of the material. This effect is also observed for the cells presented here. Without activation, the capacity obtained for the first cycle is consid- erably lower. It may slowly rise during the following cycles, but even the maximum capacity – typically reached within the first 50 cycles – is lower than that of similar cells after activation (data not shown). Therefore, a first activation (or “formation”) cycle is run with a different protocol to access the full capacity. Cyclic voltammetry was used to optimize this activation protocol. In general higher voltages, higher temperature, slower charge rates, and longer rest times at elevated voltage can increase material utilization during the activation cycle. To begin with, a very simple approach is tested: The cell is charged up to 4.0 V, i.e. to the edge of the window of electrochemical stability of the electrolyte (cf. Tab. 3.2). Starting from the pristine (discharged) state, the cell was cycled three times at a fixed rate of 25 μV/s in the range of 1.5–4.0 V, see Fig. 3.15. 150 100 50 0 -50 -100 Figure 3.15: The first three cycles of a cell. Voltage sweep rate: 25 μV/s. Charging up to 4.0V is needed to activate all of the Li2S. Only during the first cycle, however, a significant amount of charge is passed above 2.8 V. Since the voltage changes quasistatically and with a constant sweep rate, peaks in Fig. 3.15 correspond to voltage plateaus in a galvanostatic discharge profile, as in Fig. 3.14. During the discharging step, two cathodic peaks are apparent which are well separated, with a small peak or shoulder in between. After activation, i.e. for the second and third cycle, two anodic peaks are detected during the charging step, which are relatively close to each other and overlap significantly. Since in this representation, one peak corresponds to one electrochemical reaction (or one set of simultaneously 1st 2nd 3rd 54 1.5 2 2.5 3 3.5 4 Voltage vs. Li/Li+ / V Current / μAPDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |