
PDF Publication Title:
Text from PDF Page: 057
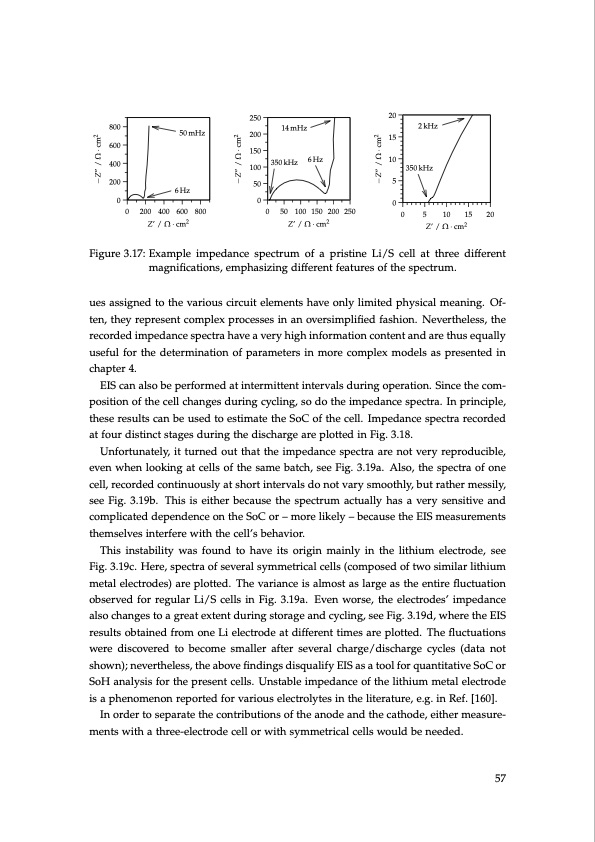
20 15 10 5 0 0 5 10 15 20 Z’ / Ω·cm2 Figure 3.17: Example impedance spectrum of a pristine Li/S cell at three different magnifications, emphasizing different features of the spectrum. ues assigned to the various circuit elements have only limited physical meaning. Of- ten, they represent complex processes in an oversimplified fashion. Nevertheless, the recorded impedance spectra have a very high information content and are thus equally useful for the determination of parameters in more complex models as presented in chapter 4. EIS can also be performed at intermittent intervals during operation. Since the com- position of the cell changes during cycling, so do the impedance spectra. In principle, these results can be used to estimate the SoC of the cell. Impedance spectra recorded at four distinct stages during the discharge are plotted in Fig. 3.18. Unfortunately, it turned out that the impedance spectra are not very reproducible, even when looking at cells of the same batch, see Fig. 3.19a. Also, the spectra of one cell, recorded continuously at short intervals do not vary smoothly, but rather messily, see Fig. 3.19b. This is either because the spectrum actually has a very sensitive and complicated dependence on the SoC or – more likely – because the EIS measurements themselves interfere with the cell’s behavior. This instability was found to have its origin mainly in the lithium electrode, see Fig. 3.19c. Here, spectra of several symmetrical cells (composed of two similar lithium metal electrodes) are plotted. The variance is almost as large as the entire fluctuation observed for regular Li/S cells in Fig. 3.19a. Even worse, the electrodes’ impedance also changes to a great extent during storage and cycling, see Fig. 3.19d, where the EIS results obtained from one Li electrode at different times are plotted. The fluctuations were discovered to become smaller after several charge/discharge cycles (data not shown); nevertheless, the above findings disqualify EIS as a tool for quantitative SoC or SoH analysis for the present cells. Unstable impedance of the lithium metal electrode is a phenomenon reported for various electrolytes in the literature, e.g. in Ref. [160]. In order to separate the contributions of the anode and the cathode, either measure- ments with a three-electrode cell or with symmetrical cells would be needed. 800 600 400 200 250 200 150 100 50 50 mHz 6Hz 00 0 200 400 600 800 0 50 100150200250 Z’ / Ω·cm2 14 mHz 350 kHz 6 Hz 2 kHz 350 kHz Z’ / Ω·cm2 57 −Z” / Ω · cm2 −Z” / Ω · cm2 −Z” / Ω · cm2PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |