
PDF Publication Title:
Text from PDF Page: 062
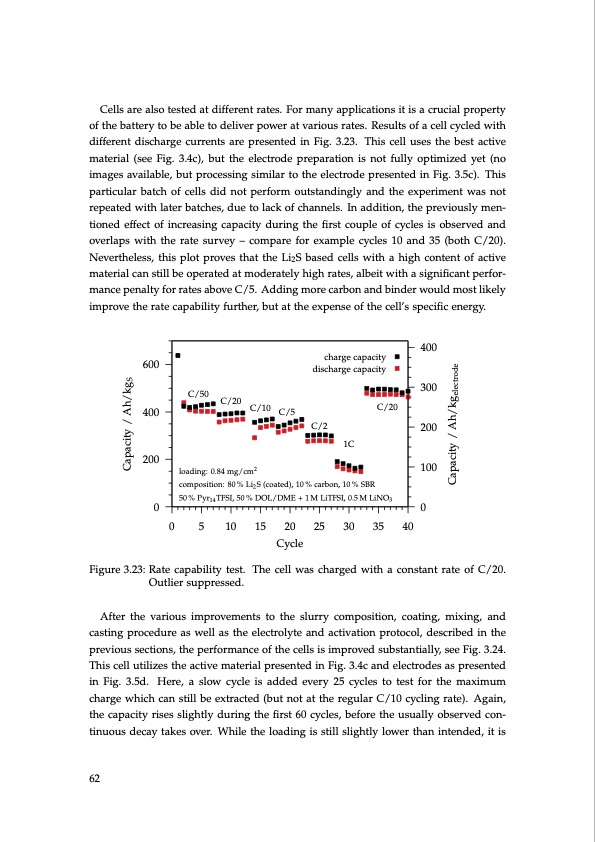
Cells are also tested at different rates. For many applications it is a crucial property of the battery to be able to deliver power at various rates. Results of a cell cycled with different discharge currents are presented in Fig. 3.23. This cell uses the best active material (see Fig. 3.4c), but the electrode preparation is not fully optimized yet (no images available, but processing similar to the electrode presented in Fig. 3.5c). This particular batch of cells did not perform outstandingly and the experiment was not repeated with later batches, due to lack of channels. In addition, the previously men- tioned effect of increasing capacity during the first couple of cycles is observed and overlaps with the rate survey – compare for example cycles 10 and 35 (both C/20). Nevertheless, this plot proves that the Li2S based cells with a high content of active material can still be operated at moderately high rates, albeit with a significant perfor- mance penalty for rates above C/5. Adding more carbon and binder would most likely improve the rate capability further, but at the expense of the cell’s specific energy. charge capacity discharge capacity C/20 C/2 1C loading: 0.84 mg/cm2 composition: 80 % Li2 S (coated), 10 % carbon, 10 % SBR 50 % Pyr14 TFSI, 50 % DOL/DME + 1 M LiTFSI, 0.5 M LiNO3 C/50 C/20 C/10 C/5 600 400 200 400 300 200 100 00 0 5 10 15 20 25 30 35 40 Cycle Figure 3.23: Rate capability test. The cell was charged with a constant rate of C/20. Outlier suppressed. After the various improvements to the slurry composition, coating, mixing, and casting procedure as well as the electrolyte and activation protocol, described in the previous sections, the performance of the cells is improved substantially, see Fig. 3.24. This cell utilizes the active material presented in Fig. 3.4c and electrodes as presented in Fig. 3.5d. Here, a slow cycle is added every 25 cycles to test for the maximum charge which can still be extracted (but not at the regular C/10 cycling rate). Again, the capacity rises slightly during the first 60 cycles, before the usually observed con- tinuous decay takes over. While the loading is still slightly lower than intended, it is 62 Capacity / Ah/kgS Capacity / Ah/kgelectrodePDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |