
PDF Publication Title:
Text from PDF Page: 101
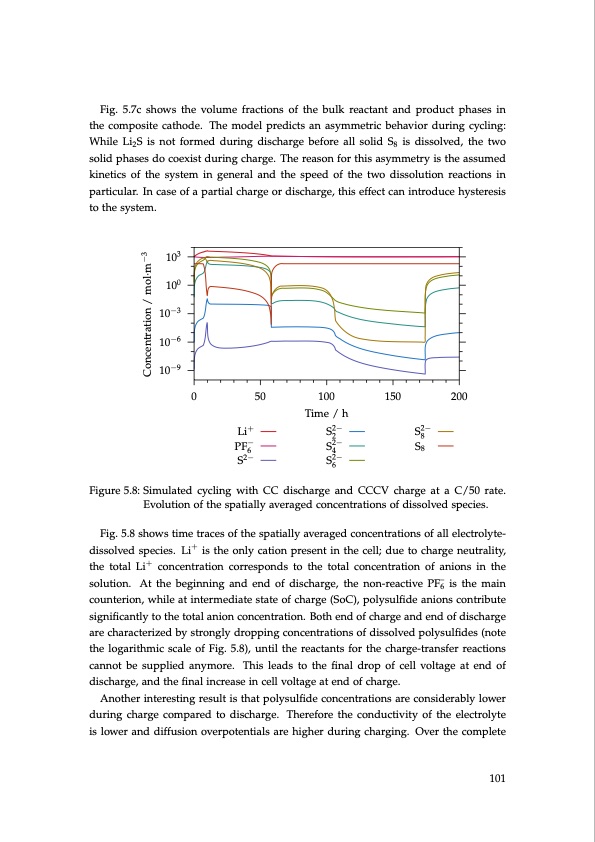
Fig. 5.7c shows the volume fractions of the bulk reactant and product phases in the composite cathode. The model predicts an asymmetric behavior during cycling: While Li2S is not formed during discharge before all solid S8 is dissolved, the two solid phases do coexist during charge. The reason for this asymmetry is the assumed kinetics of the system in general and the speed of the two dissolution reactions in particular. In case of a partial charge or discharge, this effect can introduce hysteresis to the system. 103 100 10−3 10−6 10−9 0 50 100 150 200 Time / h Li+ S2− S2− 28 PF− S2− S8 64 S2− S2− 6 Figure 5.8: Simulated cycling with CC discharge and CCCV charge at a C/50 rate. Evolution of the spatially averaged concentrations of dissolved species. Fig. 5.8 shows time traces of the spatially averaged concentrations of all electrolyte- dissolved species. Li+ is the only cation present in the cell; due to charge neutrality, the total Li+ concentration corresponds to the total concentration of anions in the solution. At the beginning and end of discharge, the non-reactive PF–6 is the main counterion, while at intermediate state of charge (SoC), polysulfide anions contribute significantly to the total anion concentration. Both end of charge and end of discharge are characterized by strongly dropping concentrations of dissolved polysulfides (note the logarithmic scale of Fig. 5.8), until the reactants for the charge-transfer reactions cannot be supplied anymore. This leads to the final drop of cell voltage at end of discharge, and the final increase in cell voltage at end of charge. Another interesting result is that polysulfide concentrations are considerably lower during charge compared to discharge. Therefore the conductivity of the electrolyte is lower and diffusion overpotentials are higher during charging. Over the complete 101 Concentration / mol·m−3PDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |