
PDF Publication Title:
Text from PDF Page: 110
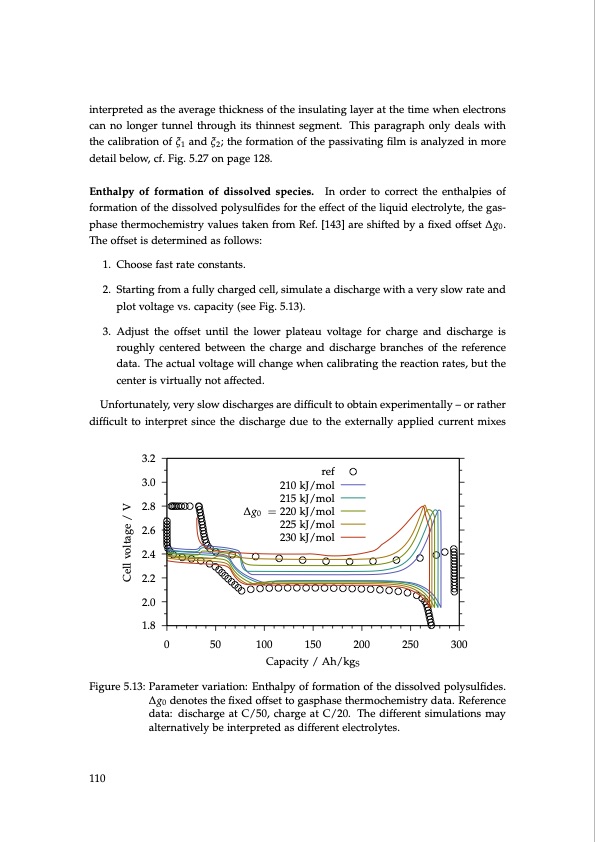
interpreted as the average thickness of the insulating layer at the time when electrons can no longer tunnel through its thinnest segment. This paragraph only deals with the calibration of ξ1 and ξ2; the formation of the passivating film is analyzed in more detail below, cf. Fig. 5.27 on page 128. Enthalpy of formation of dissolved species. In order to correct the enthalpies of formation of the dissolved polysulfides for the effect of the liquid electrolyte, the gas- phase thermochemistry values taken from Ref. [143] are shifted by a fixed offset ∆g0. The offset is determined as follows: 1. Choose fast rate constants. 2. Starting from a fully charged cell, simulate a discharge with a very slow rate and plot voltage vs. capacity (see Fig. 5.13). 3. Adjust the offset until the lower plateau voltage for charge and discharge is roughly centered between the charge and discharge branches of the reference data. The actual voltage will change when calibrating the reaction rates, but the center is virtually not affected. Unfortunately, very slow discharges are difficult to obtain experimentally – or rather difficult to interpret since the discharge due to the externally applied current mixes 3.2 3.0 2.8 2.6 2.4 2.2 2.0 1.8 Figure 5.13: Parameter variation: Enthalpy of formation of the dissolved polysulfides. ∆g0 denotes the fixed offset to gasphase thermochemistry data. Reference data: discharge at C/50, charge at C/20. The different simulations may alternatively be interpreted as different electrolytes. ref 210 kJ/mol 215 kJ/mol ∆g0 = 220 kJ/mol 225 kJ/mol 230 kJ/mol 110 0 50 100 150 200 250 300 Capacity / Ah/kgS Cell voltage / VPDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |