
PDF Publication Title:
Text from PDF Page: 007
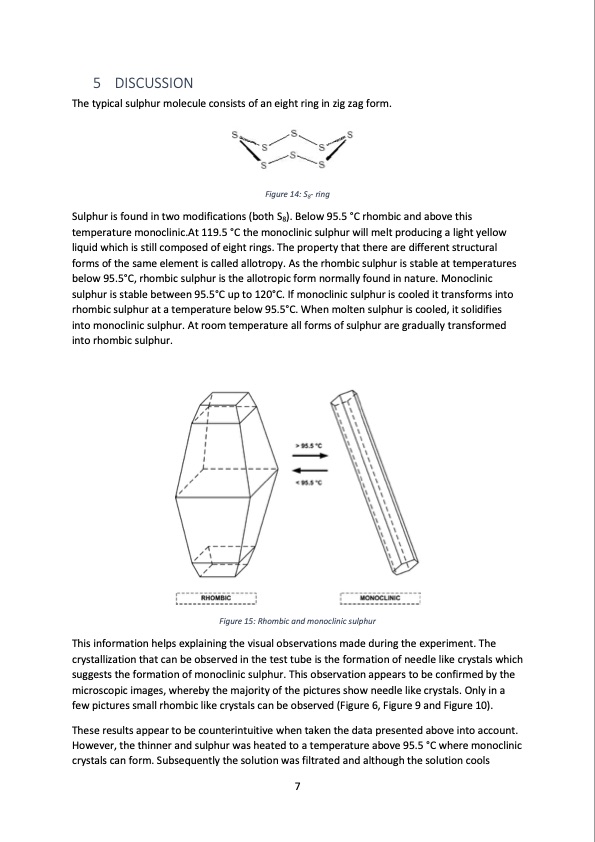
5 DISCUSSION The typical sulphur molecule consists of an eight ring in zig zag form. Figure 14: S8- ring Sulphur is found in two modifications (both S8). Below 95.5 °C rhombic and above this temperature monoclinic.At 119.5 °C the monoclinic sulphur will melt producing a light yellow liquid which is still composed of eight rings. The property that there are different structural forms of the same element is called allotropy. As the rhombic sulphur is stable at temperatures below 95.5°C, rhombic sulphur is the allotropic form normally found in nature. Monoclinic sulphur is stable between 95.5°C up to 120°C. If monoclinic sulphur is cooled it transforms into rhombic sulphur at a temperature below 95.5°C. When molten sulphur is cooled, it solidifies into monoclinic sulphur. At room temperature all forms of sulphur are gradually transformed into rhombic sulphur. Figure 15: Rhombic and monoclinic sulphur This information helps explaining the visual observations made during the experiment. The crystallization that can be observed in the test tube is the formation of needle like crystals which suggests the formation of monoclinic sulphur. This observation appears to be confirmed by the microscopic images, whereby the majority of the pictures show needle like crystals. Only in a few pictures small rhombic like crystals can be observed (Figure 6, Figure 9 and Figure 10). These results appear to be counterintuitive when taken the data presented above into account. However, the thinner and sulphur was heated to a temperature above 95.5 °C where monoclinic crystals can form. Subsequently the solution was filtrated and although the solution cools 7PDF Image | Sulphur crystals
PDF Search Title:
Sulphur crystalsOriginal File Name Searched:
rh-sulphur.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |