
PDF Publication Title:
Text from PDF Page: 011
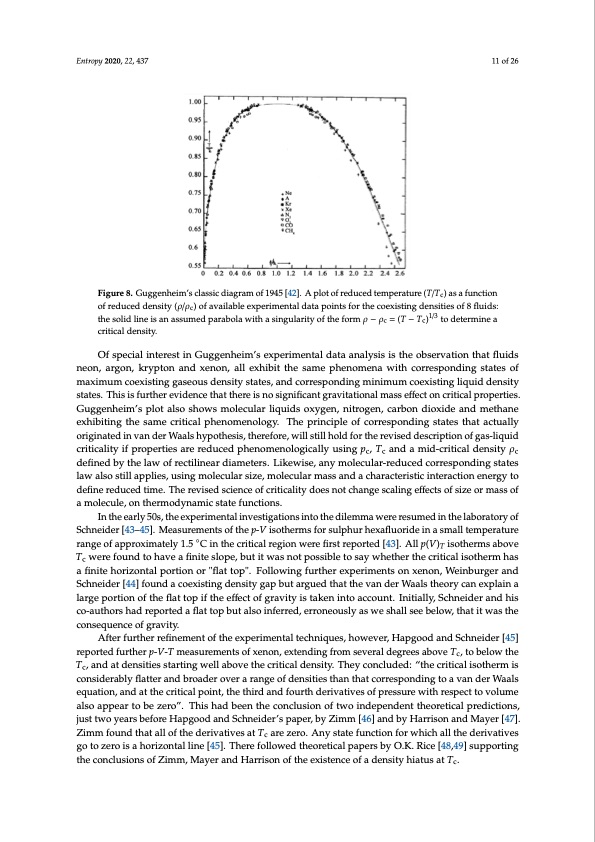
liquid phase measurements. Since there is no satisfactory scientific explanation for this hiatus, this itself is prima facie evidence for the non-existence of van der Waals continuity of liquid and gas and the non-existence of a critical point on Gibbs density surface. Ever since Guggenheim’s paper in 1945, moreover, no experimental observations of gas or liquid states of coexisting densities within the critical divide have ever been reported. In fact, the same also applies to computer studies of liquid-gas Entropy 2020, 22, 437 11 of 26 critical coexistence densities. Figure 8. Guggenheim’s classic diagram of 1945 [42]. A plot of reduced temperature (T/Tc) as a function Figure 8. Guggenheim’s classic diagram of 1945 [42]. A plot of reduced temperature (T/Tc) as a function of reduced density (ρ/ρc) of available experimental data points for the coexisting densities of 8 fluids: of reduced density (ρ/ρc) of available experimental data points for the coexisting densities of 8 fluids: the solid line is an assumed parabola with a singularity of the form ρ − ρ = (T − T )1/3 to determine a c c1/3 the solid line is an assumed parabola with a singularity of the form ρ − ρc = (T − Tc) to determine a critical density. critical density. Of special interest in Guggenheim’s experimental data analysis is the observation that fluids Of special interest in Guggenheim's experimental data analysis is the observation that fluids neon, neon, argon, krypton and xenon, all exhibit the same phenomena with corresponding states of argon, krypton and xenon, all exhibit the same phenomena with corresponding states of maximum maximum coexisting gaseous density states, and corresponding minimum coexisting liquid density coexisting gaseous density states, and corresponding minimum coexisting liquid density states. This is states. This is further evidence that there is no significant gravitational mass effect on critical properties. further evidence that there is no significant gravitational mass effect on critical properties. Guggenheim’s plot also shows molecular liquids oxygen, nitrogen, carbon dioxide and methane Guggenheim's plot also shows molecular liquids oxygen, nitrogen, carbon dioxide and methane exhibiting the same critical phenomenology. The principle of corresponding states that actually exhibiting the same critical phenomenology. The principle of corresponding states that actually originated in van der Waals hypothesis, therefore, will still hold for the revised description of gas-liquid originated in van der Waals hypothesis, therefore, will still hold for the revised description of criticality if properties are reduced phenomenologically using pc, Tc and a mid-critical density ρc gas-liquid criticality if properties are reduced phenomenologically using pc, Tc and a mid-critical defined by the law of rectilinear diameters. Likewise, any molecular-reduced corresponding states density ρc defined by the law of rectilinear diameters. Likewise, any molecular-reduced corresponding law also still applies, using molecular size, molecular mass and a characteristic interaction energy to states law also still applies, using molecular size, molecular mass and a characteristic interaction define reduced time. The revised science of criticality does not change scaling effects of size or mass of energy to define reduced time. The revised science of criticality does not change scaling effects of size a molecule, on thermodynamic state functions. or mass of a molecule, on thermodynamic state functions. In the early 50s, the experimental investigations into the dilemma were resumed in the laboratory of In the early 50s, the experimental investigations into the dilemma were resumed in the laboratory Schneider [43–45]. Measurements of the p-V isotherms for sulphur hexafluoride in a small temperature of Schneider [43–45]. Measurements of the p-V isotherms for sulphur hexafluoride in a small range of approximately 1.5 ◦C in the critical region were first reported [43]. All p(V)T isotherms above temperature range of approximately 1.5 °C in the critical region were first reported [43]. All p(V)T Tc were found to have a finite slope, but it was not possible to say whether the critical isotherm has isotherms above Tc were found to have a finite slope, but it was not possible to say whether the critical a finite horizontal portion or "flat top". Following further experiments on xenon, Weinburger and isotherm has a finite horizontal portion or "flat top". Following further experiments on xenon, Schneider [44] found a coexisting density gap but argued that the van der Waals theory can explain a Weinburger and Schneider [44] found a coexisting density gap but argued that the van der Waals large portion of the flat top if the effect of gravity is taken into account. Initially, Schneider and his theory can explain a large portion of the flat top if the effect of gravity is taken into account. Initially, co-authors had reported a flat top but also inferred, erroneously as we shall see below, that it was the Schneider and his co-authors had reported a flat top but also inferred, erroneously as we shall see consequence of gravity. below, that it was the consequence of gravity. After further refinement of the experimental techniques, however, Hapgood and Schneider [45] After further refinement of the experimental techniques, however, Hapgood and Schneider [45] reported further p-V-T measurements of xenon, extending from several degrees above Tc, to below the reported further p-V-T measurements of xenon, extending from several degrees above Tc, to below the Tc, and at densities starting well above the critical density. They concluded: “the critical isotherm is Tc, and at densities starting well above the critical density. They concluded: “the critical isotherm is considerably flatter and broader over a range of densities than that corresponding to a van der Waals considerably flatter and broader over a range of densities than that corresponding to a van der Waals equation, and at the critical point, the third and fourth derivatives of pressure with respect to volume also appear to be zero”. This had been the conclusion of two independent theoretical predictions, just two years before Hapgood and Schneider’s paper, by Zimm [46] and by Harrison and Mayer [47]. Zimm found that all of the derivatives at Tc are zero. Any state function for which all the derivatives go to zero is a horizontal line [45]. There followed theoretical papers by O.K. Rice [48,49] supporting the conclusions of Zimm, Mayer and Harrison of the existence of a density hiatus at Tc.PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |