
PDF Publication Title:
Text from PDF Page: 012
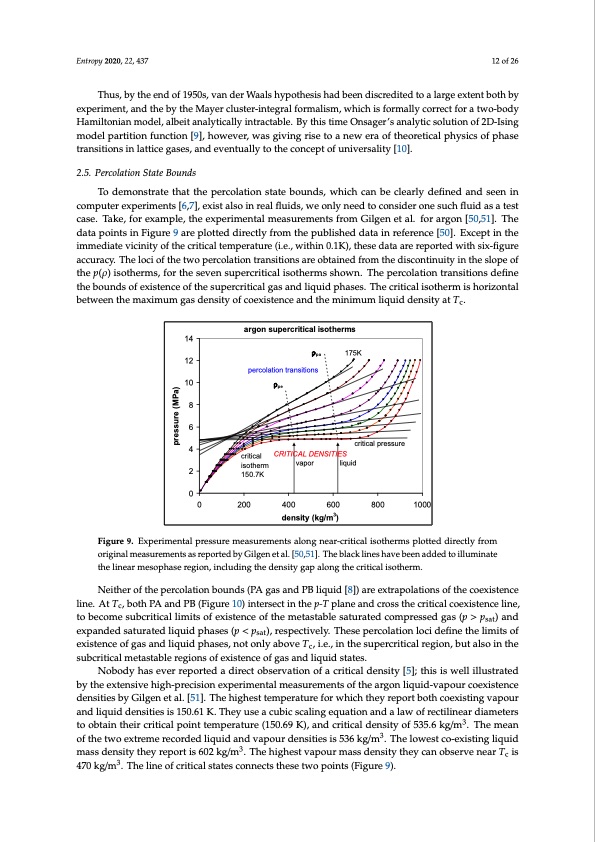
equation, and at the critical point, the third and fourth derivatives of pressure with respect to volume also appear to be zero”. This had been the conclusion of two independent theoretical predictions, just two years before Hapgood and Schneider’s paper, by Zimm [46] and by Harrison and Mayer [47]. Zimm found that all of the derivatives at Tc are zero. Any state function for which all the derivatives go Entropy 2020, 22, 437 12 of 26 to zero is a horizontal line [45]. There followed theoretical papers by O.K. Rice [48,49] supporting the conclusions of Zimm, Mayer and Harrison of the existence of a density hiatus at Tc. Thus, by the end of 1950s, van der Waals hypothesis had been discredited to a large extent both Thus, by the end of 1950s, van der Waals hypothesis had been discredited to a large extent both by by experiment, and the by the Mayer cluster-integral formalism, which is formally correct for a experiment, and the by the Mayer cluster-integral formalism, which is formally correct for a two-body two-body Hamiltonian model, albeit analytically intractable. By this time Onsager’s analytic solution Hamiltonian model, albeit analytically intractable. By this time Onsager’s analytic solution of 2D-Ising of 2D-Ising model partition function [9], however, was giving rise to a new era of theoretical physics of model partition function [9], however, was giving rise to a new era of theoretical physics of phase phase transitions in lattice gases, and eventually to the concept of universality [10]. transitions in lattice gases, and eventually to the concept of universality [10]. 22.5.5..PPerecrocolalatitoionnSStatateteBBoouunnddss TToo demonssttrraattee tthatt the percolation state bounds, which can be cclleeaarrlly ddeefifnineeddaannddseseenninin ccoomppuuteterreexxppeerrimimeennttss[[66,7,7]],,exiissttallsoinreal fflluids, we onlyneedttocconssiideerroonneessuucchhflfuluididaassaatetsetst ccaassee..TaTkake,ef,ofrorexeaxmamplpel,et,htehexepxepreimrimenetnaltamlmeaesausruemreemnetsntfsrofmromGiGlgielngeentaetl.aflo.rfaorgaorngo[5n0,[510],.5T1]h.eTdhaeta pdoaintatspoinintFsiginurFeig9uraere9 aprleotptelodtteddiredcitrleyctlfyrofmromthtehepupbulbislhisehdedddatatainin reference [50]. Except ininththee imimmeeddiaiatteeviicciiniittyofftthecriitical temperature (i.e., within 0..1K),,tthesedattaarreerreeppoorrteteddwitihthsisxix-fi-fgiguurere aacccuurraaccyy..Theellociiofftthetwopercolation transitionsareobtaiinedffrromttheedisisccoonntitninuuitiytyininththeeslsoloppeeoof f ththeepp(ρ(ρ))isisooththeermrms,s,foforrththeesesvevenensusupperecrrcirtitciaclailsiostohtehremrms shsohwown.nT.hTehpeeprceorclaotliaotniotnratnrasintisoitniosndsefdinefienthee btohuenbdosunodfsexoifsetexnisctenocfetohfethseupsueprcerrictircitailcaglagsasanadndlilqiquuididphaasseess..Thecriticaliisotthermisishhoroirziozonntatlal bbeetwtweeennththeemaaxximimuumggaassddeennssitityyooffccooeexxiisstteencceeaandttheemiiniimumlliiquiiddeenssiittyattTc. . the linear mesophase region, including the density gap along the critical isotherm. illuminate the linear mesophase region, including the density gap along the critical isotherm. Neither of the percolation bounds (PA gas and PB liquid [8]) are extrapolations of the coexistence Neither of the percolation bounds (PA gas and PB liquid [8]) are extrapolations of the coexistence line. At Tc, both PA and PB (Figure 10) intersect in the p-T plane and cross the critical coexistence line, line. At Tc, both PA and PB (Figure 10) intersect in the p-T plane and cross the critical coexistence line, to become subcritical limits of existence of the metastable saturated compressed gas (p > psat) and to become subcritical limits of existence of the metastable saturated compressed gas (p > psat) and expanded saturated liquid phases (p < psat), respectively. These percolation loci define the limits of expanded saturated liquid phases (p < psat), respectively. These percolation loci define the limits of existence of gas and liquid phases, not only above Tc, i.e., in the supercritical region, but also in the existence of gas and liquid phases, not only above Tc, i.e., in the supercritical region, but also in the subcritical metastable regions of existence of gas and liquid states. subcritical metastable regions of existence of gas and liquid states. Nobody has ever reported a direct observation of a critical density [5]; this is well illustrated by the extensive high-precision experimental measurements of the argon liquid-vapour coexistence densities by Gilgen et al. [51]. The highest temperature for which they report both coexisting vapour and liquid densities is 150.61 K. They use a cubic scaling equation and a law of rectilinear diameters to obtain their critical point temperature (150.69 K), and critical density of 535.6 kg/m3. The mean of the two extreme recorded liquid and vapour densities is 536 kg/m3. The lowest co-existing liquid mass density they report is 602 kg/m3. The highest vapour mass density they can observe near Tc is 470 kg/m3. The line of critical states connects these two points (Figure 9). c 14 12 10 8 6 4 2 0 argon supercritical isotherms ρpa 175K percolation transitions critical isotherm 150.7K vapor liquid ρpe CRITICAL DENSITIES critical pressure 0 200 400 600 800 1000 density (kg/m3) Figure 9. Experimental pressure measurements along near-critical isotherms plotted directly from Figure 9. Experimental pressure measurements along near-critical isotherms plotted directly from original measurements as reported by Gilgen et al. [50,51]. The black lines have been added to illuminate original measurements as reported by Gilgen et al. [50,51]. The black lines have been added to pressure (MPa)PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |