
PDF Publication Title:
Text from PDF Page: 017
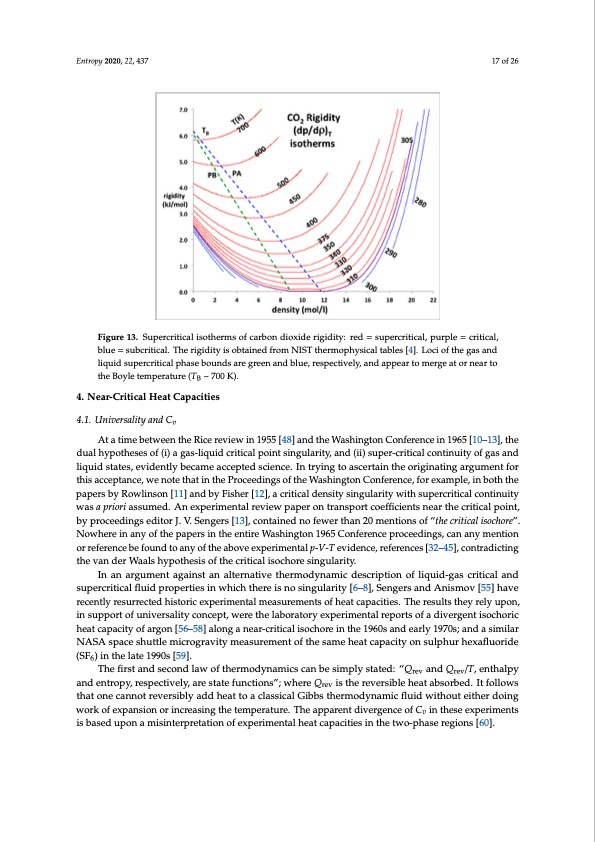
thEnrotruopgyh20z2e0,r2o2,r4e3m7 ains unknown. The same behaviour is seen in the rigidity plots for other liqu1id7 so,f 2fo6 r example water [54]. Figure 13. Supercritical isotherms of carbon dioxide rigidity: red = supercritical, purple = critical, blue = Figure 13. Supercritical isotherms of carbon dioxide rigidity: red = supercritical, purple = critical, subcritical. The rigidity is obtained from NIST thermophysical tables [4]. Loci of the gas and liquid blue = subcritical. The rigidity is obtained from NIST thermophysical tables [4]. Loci of the gas and supercritical phase bounds are green and blue, respectively, and appear to merge at or near to the Boyle liquid supercritical phase bounds are green and blue, respectively, and appear to merge at or near to temperature (TB ~ 700 K). the Boyle temperature (TB ~ 700 K). fluiqidusidhsatvaeterse,qeuviirdeednetlxypboenceanmtseda=cc3eptote4d, oscrievnecne.hIinghtreyrin[5g4]toinasocredretaritnotphaeraomrigeitneraitzineganaragpupmareennttffolrat tothpiswacicthepintanthce, wexepneortime tehnatailn uthnecPertoacienetdy.inTgsheof pthresWenatsahtiinogntoonfCnoenafer-rcernictiec,afloreexxpaemripmle,nitnalbortehsuthltes, hpoawpevrserb,yhRasowbeleinsaodnv[e1r1s]ealyndafbfeycFteisdhbery[h1y2]p,oathcreistiecsa,lwdhenicshitayrseinhgeurelasreiteynwtoitbhesiunpceorrcreitcitcianl cthonetliinguhityof ewxpaesraimpreinotrailadssautam,eadn.dAhnenecxepmerismrepnrteaslernetvtihewecpriatpicearlodnivtirdaenasptoTrctacnodeffithceiesnutpsenrcerairtitchaelmcreitsiocaplhpaosein.t, 4. Near-Critical Heat Capacities Equation-of-state experimental data, albeit accurate and painstakingly obtained, may not be the most reliable to decide the issue of critical flatness. It is not easy to distinguish a low curvature region 4.1. Universality and Cv from one that is in fact a straight line in p(ρ)T. The literature critical-point universality theory predicts d that thAettaemtimperbaetutwreeoenr pthresRsuicreersecvaileews ains Δ19ρ55a[l4o8n]gatnhdetchreitWicalsihsiontghteornmC,ownhfeicrhenccoeuilnd1t9h6e5re[1fo0r–e13a]p,ptheear todubael hvyepryotfhleasteasnoyfw(ia)ya gwaist-hliqnutidhecrhityicpaoltphoeisnist.sMingaunlyarpit-yV,-aTndex(piie)rsiumpenr-tcarlitriecsaul lctosnftoinrurietyalomf goalsecaunldar by proceedings editor J. V. Sengers [13], contained no fewer than 20 mentions of “the critical isochore”. 4. Near-Critical Heat Capacities Nowhere in any of the papers in the entire Washington 1965 Conference proceedings, can any mention or reference be found to any of the above experimental p-V-T evidence, references [32–45], contradicting 4.1. Universality and Cv 17 of 27 the van der Waals hypothesis of the critical isochore singularity. In an argument against an alternative thermodynamic description of liquid-gas critical and At a time between the Rice review in 1955 [48] and the Washington Conference in 1965 [10–13], supercritical fluid properties in which there is no singularity [6–8], Sengers and Anismov [55] have the dual hypotheses of (i) a gas-liquid critical point singularity, and (ii) super-critical continuity of gas recently resurrected historic experimental measurements of heat capacities. The results they rely upon, and liquid states, evidently became accepted science. In trying to ascertain the originating argument in support of universality concept, were the laboratory experimental reports of a divergent isochoric for this acceptance, we note that in the Proceedings of the Washington Conference, for example, in heat capacity of argon [56–58] along a near-critical isochore in the 1960s and early 1970s; and a similar both the papers by Rowlinson [11] and by Fisher [12], a critical density singularity with supercritical NASA space shuttle microgravity measurement of the same heat capacity on sulphur hexafluoride continuity was a priori assumed. An experimental review paper on transport coefficients near the (SF ) in the late 1990s [59]. critic6al point, by proceedings editor J. V. Sengers [13], contained no fewer than 20 mentions of “the The first and second law of thermodynamics can be simply stated: “Q and Q /T, enthalpy critical isochore”. Nowhere in any of the papers in the entire Washington 1965rCevonferenrceev proceedings, and entropy, respectively, are state functions”; where Q is the reversible heat absorbed. It follows can any mention or reference be found to any of the abroeve experimental p-V-T evidence, references that one cannot reversibly add heat to a classical Gibbs thermodynamic fluid without either doing [32–45], contradicting the van der Waals hypothesis of the critical isochore singularity. workofexpansionorincreasingthetemperature.TheapparentdivergenceofC intheseexperiments In an argument against an alternative thermodynamic description of lviquid-gas critical and is based upon a misinterpretation of experimental heat capacities in the two-phase regions [60]. supercritical fluid properties in which there is no singularity [6–8], Sengers and Anismov [55] have recently resurrected historic experimental measurements of heat capacities. The results they rely upon,PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |