
PDF Publication Title:
Text from PDF Page: 019
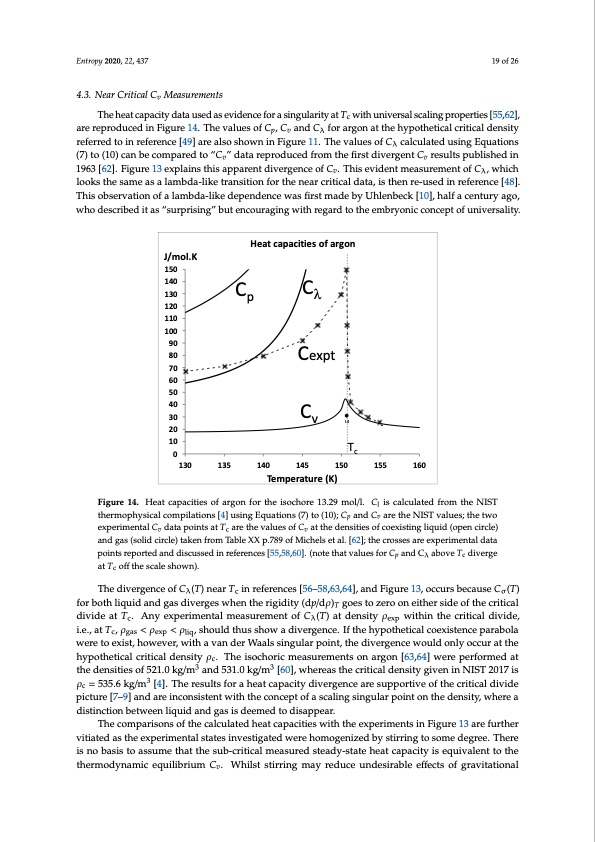
Entropy 2020, 22, 437 19 of 26 4.3. Near Critical Cv Measurements The heat capacity data used as evidence for a singularity at Tc with universal scaling properties [55,62], are reproduced in Figure 14. The values of Cp, Cv and Cλ for argon at the hypothetical critical density referred to in reference [49] are also shown in Figure 11. The values of Cλ calculated using Equations (7) to (10) can be compared to “Cv” data reproduced from the first divergent Cv results published in 1963 [62]. Figure 13 explains this apparent divergence of Cv. This evident measurement of Cλ, which looks the same as a lambda-like transition for the near critical data, is then re-used in reference [48]. This observation of a lambda-like dependence was first made by Uhlenbeck [10], half a century ago, who described it as “surprising” but encouraging with regard to the embryonic concept of universality. J/mol.K 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0 Heat capacities of argon Cλ Cexpt Cv Cp Tc 130 135 140 Temperature (K) 145 150 155 160 FFigiguurere1144.. HHeaetatccaappaacictiteisesooffaarrgon for the isochore 13..29 mooll//l.l. Cl is callcullatted ffrroomththeeNNISISTT l ththeremrmopophhysyisciaclalcocommppilialtaitoionnss[4[4]]ussiingEquatiions (7)tto1(100););CppaandCv are the NISTvvaallueess;;ththeetwtwoo exexppereirmimeenntatallCCvvddaatatappooinintstsaattTccaarreettheevalluesofCvatthedensities ofcoexistiinglliiquiid((oopeenncciricrclele)) ananddggaass(s(soolildidccirircclele))ttaakkeenffrrom Table XX p.789 of Michels et all.. [[62]];; ttheccrrossseessaarreeeexxppeerirmimeenntatal lddaatata points reported and discussed in references [55,58,60]. (note that values for C and C above T diverge points reported and discussed in references [55,58,60]. (note that values for Cp p and Cλλ above Tc c diverge atT offthescaleshown). at Tc coff the scale shown). ThedivergenceofC (T)nearT inreferences[56–58,63,64],andFigure13,occursbecauseC (T) λcσ It appears that the surface energy effect on heat capacity measurement cancels to some degree the for both liquid and gas diverges when the rigidity (dp/dρ) enthalpy of evaporation (liq → vap) given by ΔHv in Equation (5) below Tc. The surface work is of divide at T . Any experimental measurement of C (T) at density ρ within the critical divide, c λexp opposite sign, so the stirred heat capacities in the vicinity of Tc are in-between Cλ and Cv with a weak < ρ divergence at Tc as seen in Figure 14. The pointed peak at around 40 J/K·mol in the isochoric heat < ρ , should thus show a divergence. If the hypothetical coexistence parabola were to exist, however, with a van der Waals singular point, the divergence would only occur at the i.e., at T , ρ c gas exp liq capacity at Tc is explained by an increase in surface tension and heterophase fluctuations starting hypothetical critical density ρ . The isochoric measurements on argon [63,64] were performed at c around 140 K in the subcritical 2-phase range as Tc is approached. For T > Tc, Cv returns to the lower the densities of 521.0 kg/m3 and 531.0 kg/m3 [60], whereas the critical density given in NIST 2017 is liquid-state value on exiting the mesophase at higher temperatures around 175K (see Figure 11). ρc = 535.6 kg/m3 [4]. The results for a heat capacity divergence are supportive of the critical divide picture [7–9] and are inconsistent with the concept of a scaling singular point on the density, where a 4.4. Space Shuttle Experiments distinction between liquid and gas is deemed to disappear. This also applies to the micro-gravity experimental measurement of a heat capacity for SF6 along a The comparisons of the calculated heat capacities with the experiments in Figure 13 are further near critical isochore aboard the NASA space shuttle [59]. The publicly available data points of the vitiated as the experimental states investigated were homogenized by stirring to some degree. There NASA Space shuttle experiment are shown in Figure 15 alongside the heat capacities Cp, Cv and Cλ [4]. is no basis to assume that the sub-critical measured steady-state heat capacity is equivalent to the A comparison shows that what was actually measured in the space-shuttle experiment (labelled Css) thermodynamic equilibrium Cv. Whilst stirring may reduce undesirable effects of gravitational relates to Cλ a few degrees below Tc, and to Cv a few degrees above Tc, but is possibly made to look like a lambda-transition in the vicinity of Tc by the stirring or steady-state emulsification. T goes to zero on either side of the critical 20 of 27PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |