
PDF Publication Title:
Text from PDF Page: 020
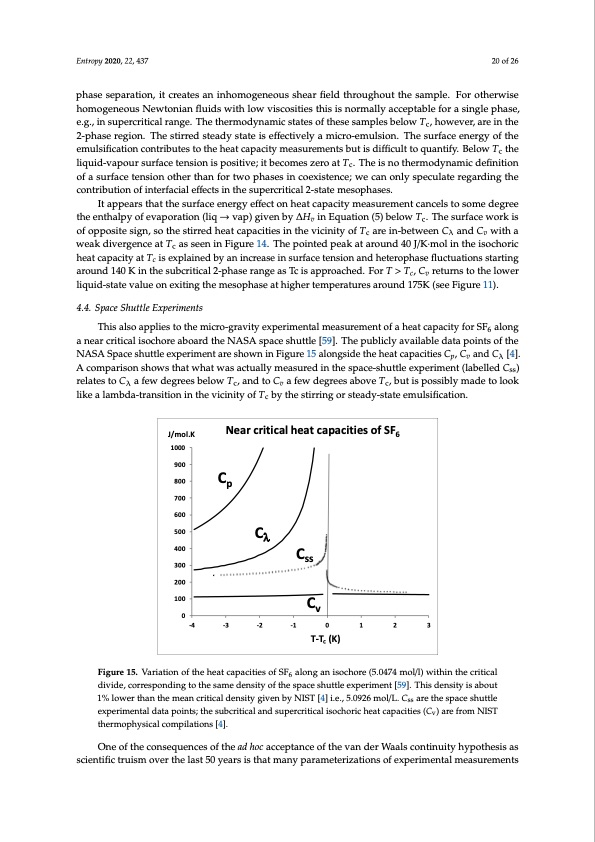
Entropy 2020, 22, 437 20 of 26 phase separation, it creates an inhomogeneous shear field throughout the sample. For otherwise homogeneous Newtonian fluids with low viscosities this is normally acceptable for a single phase, e.g., in supercritical range. The thermodynamic states of these samples below Tc, however, are in the 2-phase region. The stirred steady state is effectively a micro-emulsion. The surface energy of the emulsification contributes to the heat capacity measurements but is difficult to quantify. Below Tc the liquid-vapour surface tension is positive; it becomes zero at Tc. The is no thermodynamic definition of a surface tension other than for two phases in coexistence; we can only speculate regarding the contribution of interfacial effects in the supercritical 2-state mesophases. It appears that the surface energy effect on heat capacity measurement cancels to some degree the enthalpy of evaporation (liq → vap) given by ∆Hv in Equation (5) below Tc. The surface work is of opposite sign, so the stirred heat capacities in the vicinity of Tc are in-between Cλ and Cv with a weak divergence at Tc as seen in Figure 14. The pointed peak at around 40 J/K·mol in the isochoric heat capacity at Tc is explained by an increase in surface tension and heterophase fluctuations starting around 140 K in the subcritical 2-phase range as Tc is approached. For T > Tc, Cv returns to the lower liquid-state value on exiting the mesophase at higher temperatures around 175K (see Figure 11). 4.4. Space Shuttle Experiments This also applies to the micro-gravity experimental measurement of a heat capacity for SF6 along a near critical isochore aboard the NASA space shuttle [59]. The publicly available data points of the NASA Space shuttle experiment are shown in Figure 15 alongside the heat capacities Cp, Cv and Cλ [4]. A comparison shows that what was actually measured in the space-shuttle experiment (labelled Css) relates to Cλ a few degrees below Tc, and to Cv a few degrees above Tc, but is possibly made to look like a lambda-transition in the vicinity of Tc by the stirring or steady-state emulsification. 21 of 27 J/mol.K 1000 900 800 700 600 500 400 300 200 100 Near critical heat capacities of SF6 Cp Cλ Css 0 -4 -3 -2 -1 0 1 2 3 T-Tc (K) Cv FFigiguurere1155..VVaarriaiatitoionnoffttheheatcapacitiesofSF6 alonganiisochore((55..0474mool/l/l)l)witihthininththeecrcirtitciaclal 6 ddivividide,ec,ocorrersepspoonnddininggtotothtehesasmameedednesnistyityofotfhtehsepsapcaecsehsuhtutltetlexepxepreimrimenetn[t5[95]9.T].hTishdisednesnitsyitiysaisbaobuotu1t% lo1w%elrowthearnththane tmheamnecarnitcicraitlicdaelndseintysitgyivgeinvebnybyNNISITST[4[]4]i.ie.e.,.,55.0.0992266mol/L. Css are tthessppaacceeshshuutttltele exexppereirmimeenntatallddaatatappooininttss;;tthesubcriiticaland supercriticalisochoriicheattccaapaaccititieiess(C(Cvv))aarreefrforomNNISISTT ththeremrmoopphhyysisciacallcocommppilialatitoionnss[4[4].]. One of the consequences of the ad hoc acceptance of the van der Waals continuity hypothesis as One of the consequences of the ad hoc acceptance of the van der Waals continuity hypothesis as scientific truism over the last 50 years is that many parameterizations of experimental measurements scientific truism over the last 50 years is that many parameterizations of experimental measurements of thermodynamic properties have been prejudiced by the a priori assumption of a singularity that does not exist. The adoption of a singular point scaling parabola to interpolate the data between ρc(gas) and ρc(liq) at Tc, with an intermediate singularity, is widespread and misleading. It leads to unreal values in the vicinity of Tc. An example in the present context is the misinterpretation of SF6 space shuttle data. The real thermodynamic Cv data for SF6 are the NIST values. An equation-of-state proposed by Kostrowicka-Wyczalkowska and Sengers [65] assumes a scaling singularity at the outset,PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |