
PDF Publication Title:
Text from PDF Page: 023
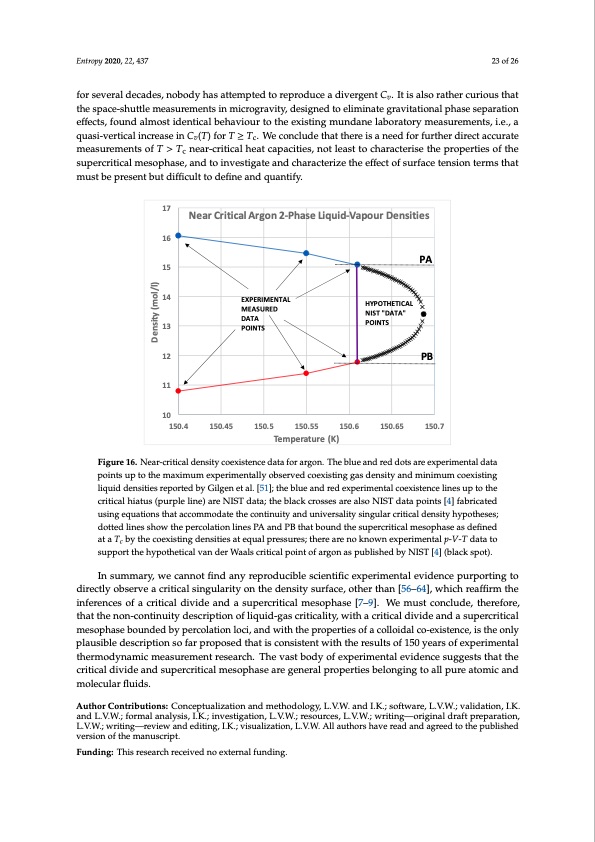
point. Thus, both pure gas and pure liquid states coexist at the same T,p states with uniform chemical potential throughout at equilibrium in the mesophase. Looking at the experimental data, (Figures 9–11) it appears that the mesophase may extend all the way to low density at the Boyle temperature, narrowing the density gap as it does so. Comparing critical point hypothesis with experiment establishes a scientific truth. The data Entropy 2020, 22, 437 23 of 26 described as “experiment” in reference [4], i.e., as obtained from NIST tabulations, are based upon the TSW-equation-of-state [68] with hypothetical “crossover equations” [65] near Tc. In the region of the critical divide and the immediate supercritical region, data points are either fabricated by hypothetical for several decades, nobody has attempted to reproduce a divergent Cv. It is also rather curious that scaling equations, or distorted by the continuity hypothesis implicit in the equations-of-state used. the space-shuttle measurements in microgravity, designed to eliminate gravitational phase separation These multiparameter equations tell us nothing about the underlying science of critical and effects, found almost identical behaviour to the existing mundane laboratory measurements, i.e., a supercritical state bounds; they produce hypothetical near-critical and supercritical mesophase data quasi-vertical increase in Cv(T) for T ≥ Tc. We conclude that there is a need for further direct accurate that have never been experimentally measured. Figure 16 shows how the scientific malpractice of measurements of T > Tc near-critical heat capacities, not least to characterise the properties of the assuming the van der Waals and universal scaling hypotheses to be established scientific truth, leads supercritical mesophase, and to investigate and characterize the effect of surface tension terms that to the spurious experimental p-V-T data [4,19] in the vicinity of Tc. must be present but difficult to define and quantify. 17 16 15 14 13 12 11 10 150.4 150.45 150.5 150.55 150.6 150.65 Temperature (K) 150.7 Near Critical Argon 2-Phase Liquid-Vapour Densities PA EXPERIMENTAL MEASURED DATA HYPOTHETICAL NIST "DATA" POINTS POINTS PB Figure 16. Near-critical density coexistence data for argon. The blue and red dots are experimental data Figure 16. Near-critical density coexistence data for argon. The blue and red dots are experimental data points up to the maximum experimentally observed coexisting gas density and minimum coexisting points up to the maximum experimentally observed coexisting gas density and minimum coexisting liquid densities reported by Gilgen et al. [51]; the blue and red experimental coexistence lines up to the liquid densities reported by Gilgen et al. [51]; the blue and red experimental coexistence lines up to the critical hiatus (purple line) are NIST data; the black crosses are also NIST data points [4] fabricated critical hiatus (purple line) are NIST data; the black crosses are also NIST data points [4] fabricated using equations that accommodate the continuity and universality singular critical density hypotheses; using equations that accommodate the continuity and universality singular critical density hypotheses; dotted lines show the percolation lines PA and PB that bound the supercritical mesophase as defined dotted lines show the percolation lines PA and PB that bound the supercritical mesophase as defined at at a Tc by the coexisting densities at equal pressures; there are no known experimental p-V-T data to a Tc by the coexisting densities at equal pressures; there are no known experimental p-V-T data to support the hypothetical van der Waals critical point of argon as published by NIST [4] (black spot). support the hypothetical van der Waals critical point of argon as published by NIST [4] (black spot). In summary, we cannot find any reproducible scientific experimental evidence purporting to Finally, it only takes one experimental property to disagree with theory for the basic hypothesis to directly observe a critical singularity on the density surface, other than [56–64], which reaffirm the be wrong. The present equation-of-state analysis with confirmation of a critical divide would appear to inferences of a critical divide and a supercritical mesophase [7–9]. We must conclude, therefore, suffice. However, there will always be those who cite the “logarithmic divergence of the isochoric heat that the non-continuity description of liquid-gas criticality, with a critical divide and a supercritical capacity on both sides of Tc” as discovered by the historic measurements of the 1960s and confirmed mesophase bounded by percolation loci, and with the properties of a colloidal co-existence, is the only plausible description so far proposed that is consistent with the results of 150 years of experimental thermodynamic measurement research. The vast body of experimental evidence suggests that the critical divide and supercritical mesophase are general properties belonging to all pure atomic and molecular fluids. Author Contributions: Conceptualization and methodology, L.V.W. and I.K.; software, L.V.W.; validation, I.K. and L.V.W.; formal analysis, I.K.; investigation, L.V.W.; resources, L.V.W.; writing—original draft preparation, L.V.W.; writing—review and editing, I.K.; visualization, L.V.W. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding. Density (mol/l)PDF Image | Supercritical Fluid Gaseous and Liquid States
PDF Search Title:
Supercritical Fluid Gaseous and Liquid StatesOriginal File Name Searched:
entropy-22-00437.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |