
PDF Publication Title:
Text from PDF Page: 054
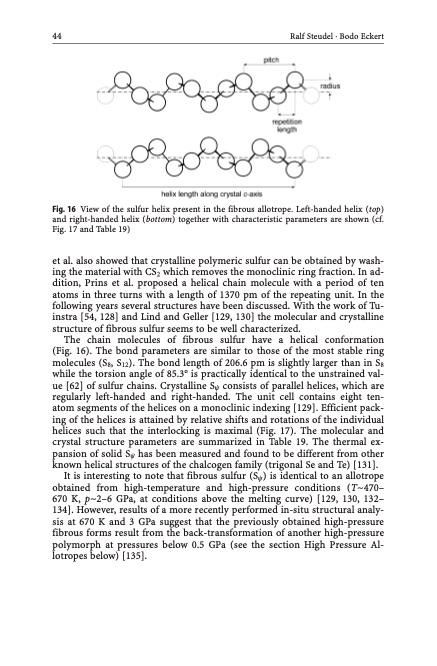
44 Ralf Steudel · Bodo Eckert Fig. 16 View of the sulfur helix present in the fibrous allotrope. Left-handed helix (top) and right-handed helix (bottom) together with characteristic parameters are shown (cf. Fig. 17 and Table 19) et al. also showed that crystalline polymeric sulfur can be obtained by wash- ing the material with CS2 which removes the monoclinic ring fraction. In ad- dition, Prins et al. proposed a helical chain molecule with a period of ten atoms in three turns with a length of 1370 pm of the repeating unit. In the following years several structures have been discussed. With the work of Tu- instra [54, 128] and Lind and Geller [129, 130] the molecular and crystalline structure of fibrous sulfur seems to be well characterized. The chain molecules of fibrous sulfur have a helical conformation (Fig. 16). The bond parameters are similar to those of the most stable ring molecules (S8, S12). The bond length of 206.6 pm is slightly larger than in S8 while the torsion angle of 85.3 is practically identical to the unstrained val- ue [62] of sulfur chains. Crystalline Sy consists of parallel helices, which are regularly left-handed and right-handed. The unit cell contains eight ten- atom segments of the helices on a monoclinic indexing [129]. Efficient pack- ing of the helices is attained by relative shifts and rotations of the individual helices such that the interlocking is maximal (Fig. 17). The molecular and crystal structure parameters are summarized in Table 19. The thermal ex- pansion of solid Sy has been measured and found to be different from other known helical structures of the chalcogen family (trigonal Se and Te) [131]. It is interesting to note that fibrous sulfur (Sy) is identical to an allotrope obtained from high-temperature and high-pressure conditions (T~470– 670 K, p~2–6 GPa, at conditions above the melting curve) [129, 130, 132– 134]. However, results of a more recently performed in-situ structural analy- sis at 670 K and 3 GPa suggest that the previously obtained high-pressure fibrous forms result from the back-transformation of another high-pressure polymorph at pressures below 0.5 GPa (see the section High Pressure Al- lotropes below) [135].PDF Image | Topics in Current Chemistry
PDF Search Title:
Topics in Current ChemistryOriginal File Name Searched:
Elemental-Sulfur-und-Sulfur-Rich-Compounds-I.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |