
PDF Publication Title:
Text from PDF Page: 112
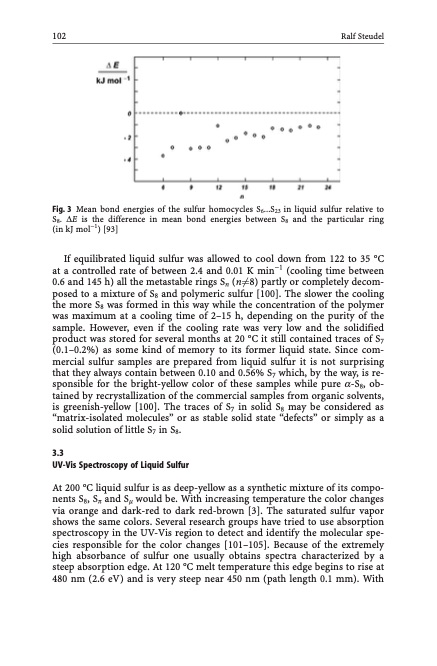
102 Ralf Steudel Fig. 3 Mean bond energies of the sulfur homocycles S6...S23 in liquid sulfur relative to S8. DE is the difference in mean bond energies between S8 and the particular ring (in kJ mol1) [93] If equilibrated liquid sulfur was allowed to cool down from 122 to 35 C at a controlled rate of between 2.4 and 0.01 K min1 (cooling time between 0.6 and 145 h) all the metastable rings Sn (n61⁄48) partly or completely decom- posed to a mixture of S8 and polymeric sulfur [100]. The slower the cooling the more S8 was formed in this way while the concentration of the polymer was maximum at a cooling time of 2–15 h, depending on the purity of the sample. However, even if the cooling rate was very low and the solidified product was stored for several months at 20 C it still contained traces of S7 (0.1–0.2%) as some kind of memory to its former liquid state. Since com- mercial sulfur samples are prepared from liquid sulfur it is not surprising that they always contain between 0.10 and 0.56% S7 which, by the way, is re- sponsible for the bright-yellow color of these samples while pure a-S8, ob- tained by recrystallization of the commercial samples from organic solvents, is greenish-yellow [100]. The traces of S7 in solid S8 may be considered as “matrix-isolated molecules” or as stable solid state “defects” or simply as a solid solution of little S7 in S8. 3.3 UV-Vis Spectroscopy of Liquid Sulfur At 200 C liquid sulfur is as deep-yellow as a synthetic mixture of its compo- nents S8, Sp and Sm would be. With increasing temperature the color changes via orange and dark-red to dark red-brown [3]. The saturated sulfur vapor shows the same colors. Several research groups have tried to use absorption spectroscopy in the UV-Vis region to detect and identify the molecular spe- cies responsible for the color changes [101–105]. Because of the extremely high absorbance of sulfur one usually obtains spectra characterized by a steep absorption edge. At 120 C melt temperature this edge begins to rise at 480 nm (2.6 eV) and is very steep near 450 nm (path length 0.1 mm). WithPDF Image | Topics in Current Chemistry
PDF Search Title:
Topics in Current ChemistryOriginal File Name Searched:
Elemental-Sulfur-und-Sulfur-Rich-Compounds-I.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |