
PDF Publication Title:
Text from PDF Page: 117
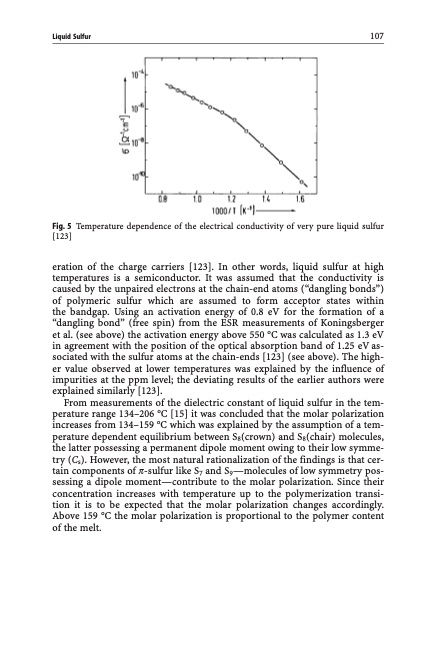
Liquid Sulfur 107 Fig. 5 Temperature dependence of the electrical conductivity of very pure liquid sulfur [123] eration of the charge carriers [123]. In other words, liquid sulfur at high temperatures is a semiconductor. It was assumed that the conductivity is caused by the unpaired electrons at the chain-end atoms (“dangling bonds”) of polymeric sulfur which are assumed to form acceptor states within the bandgap. Using an activation energy of 0.8 eV for the formation of a “dangling bond” (free spin) from the ESR measurements of Koningsberger et al. (see above) the activation energy above 550 C was calculated as 1.3 eV in agreement with the position of the optical absorption band of 1.25 eV as- sociated with the sulfur atoms at the chain-ends [123] (see above). The high- er value observed at lower temperatures was explained by the influence of impurities at the ppm level; the deviating results of the earlier authors were explained similarly [123]. From measurements of the dielectric constant of liquid sulfur in the tem- perature range 134–206 C [15] it was concluded that the molar polarization increases from 134–159 C which was explained by the assumption of a tem- perature dependent equilibrium between S8(crown) and S8(chair) molecules, the latter possessing a permanent dipole moment owing to their low symme- try (Cs). However, the most natural rationalization of the findings is that cer- tain components of p-sulfur like S7 and S9—molecules of low symmetry pos- sessing a dipole moment—contribute to the molar polarization. Since their concentration increases with temperature up to the polymerization transi- tion it is to be expected that the molar polarization changes accordingly. Above 159 C the molar polarization is proportional to the polymer content of the melt.PDF Image | Topics in Current Chemistry
PDF Search Title:
Topics in Current ChemistryOriginal File Name Searched:
Elemental-Sulfur-und-Sulfur-Rich-Compounds-I.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |