
PDF Publication Title:
Text from PDF Page: 136
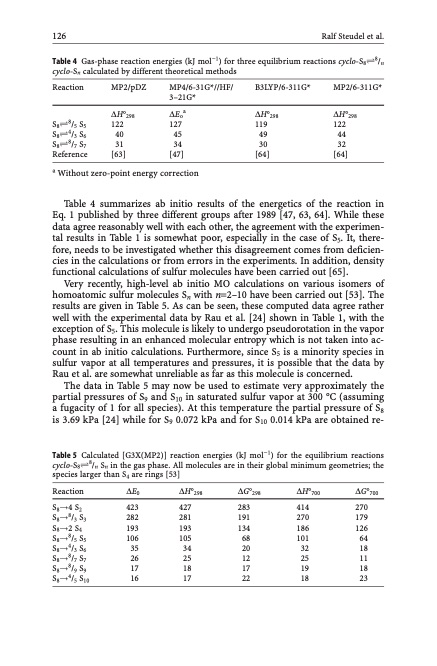
126 Ralf Steudel et al. Table 4 Gas-phase reaction energies (kJ mol1) for three equilibrium reactions cyclo-S8Ð8/n cyclo-Sn calculated by different theoretical methods Reaction S8Ð8/5 S5 S8Ð4/3 S6 S8Ð8/7 S7 Reference Table 4 summarizes ab initio results of the energetics of the reaction in Eq. 1 published by three different groups after 1989 [47, 63, 64]. While these data agree reasonably well with each other, the agreement with the experimen- tal results in Table 1 is somewhat poor, especially in the case of S5. It, there- fore, needs to be investigated whether this disagreement comes from deficien- cies in the calculations or from errors in the experiments. In addition, density functional calculations of sulfur molecules have been carried out [65]. Very recently, high-level ab initio MO calculations on various isomers of homoatomic sulfur molecules Sn with n=2–10 have been carried out [53]. The results are given in Table 5. As can be seen, these computed data agree rather well with the experimental data by Rau et al. [24] shown in Table 1, with the exception of S5. This molecule is likely to undergo pseudorotation in the vapor phase resulting in an enhanced molecular entropy which is not taken into ac- count in ab initio calculations. Furthermore, since S5 is a minority species in sulfur vapor at all temperatures and pressures, it is possible that the data by Rau et al. are somewhat unreliable as far as this molecule is concerned. The data in Table 5 may now be used to estimate very approximately the partial pressures of S9 and S10 in saturated sulfur vapor at 300 C (assuming a fugacity of 1 for all species). At this temperature the partial pressure of S8 is 3.69 kPa [24] while for S9 0.072 kPa and for S10 0.014 kPa are obtained re- Table 5 Calculated [G3X(MP2)] reaction energies (kJ mol1) for the equilibrium reactions cyclo-S8Ð8/n Sn in the gas phase. All molecules are in their global minimum geometries; the species larger than S4 are rings [53] MP2/pDZ MP4/6-31G*//HF/ B3LYP/6-311G* MP2/6-311G* 3–21G* DH298 DEoa 122 127 40 45 31 34 DH298 DH298 119 122 49 44 30 32 [64] [64] [63] [47] a Without zero-point energy correction Reaction S8!4 S2 S8!8/3 S3 S8!2 S4 S8!8/5 S5 S8!4/3 S6 S8!8/7 S7 S8!8/9 S9 S8!4/5 S10 DE0 DH298 DG298 423 427 283 282 281 191 193 193 134 106 105 68 35 34 20 26 25 12 17 18 17 16 17 22 DH700 DG700 414 270 270 179 186 126 101 64 32 18 25 11 19 18 18 23PDF Image | Topics in Current Chemistry
PDF Search Title:
Topics in Current ChemistryOriginal File Name Searched:
Elemental-Sulfur-und-Sulfur-Rich-Compounds-I.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |