
PDF Publication Title:
Text from PDF Page: 156
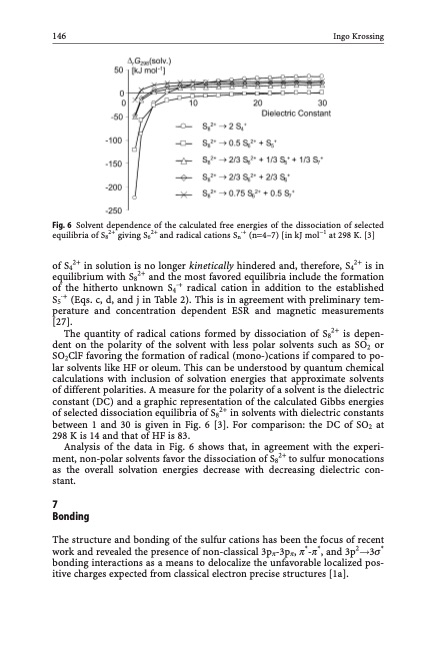
146 Ingo Krossing Fig. 6 Solvent dependence of the calculated free energies of the dissociation of selected equilibria of S82+ giving S62+ and radical cations Sn·+ (n=4–7) [in kJ mol1 at 298 K. [3] of S42+ in solution is no longer kinetically hindered and, therefore, S42+ is in equilibrium with S82+ and the most favored equilibria include the formation of the hitherto unknown S4·+ radical cation in addition to the established S5·+ (Eqs. c, d, and j in Table 2). This is in agreement with preliminary tem- perature and concentration dependent ESR and magnetic measurements [27]. The quantity of radical cations formed by dissociation of S82+ is depen- dent on the polarity of the solvent with less polar solvents such as SO2 or SO2ClF favoring the formation of radical (mono-)cations if compared to po- lar solvents like HF or oleum. This can be understood by quantum chemical calculations with inclusion of solvation energies that approximate solvents of different polarities. A measure for the polarity of a solvent is the dielectric constant (DC) and a graphic representation of the calculated Gibbs energies of selected dissociation equilibria of S82+ in solvents with dielectric constants between 1 and 30 is given in Fig. 6 [3]. For comparison: the DC of SO2 at 298 K is 14 and that of HF is 83. Analysis of the data in Fig. 6 shows that, in agreement with the experi- ment, non-polar solvents favor the dissociation of S82+ to sulfur monocations as the overall solvation energies decrease with decreasing dielectric con- stant. 7 Bonding The structure and bonding of the sulfur cations has been the focus of recent work and revealed the presence of non-classical 3pp-3pp, p*-p*, and 3p2!3s* bonding interactions as a means to delocalize the unfavorable localized pos- itive charges expected from classical electron precise structures [1a].PDF Image | Topics in Current Chemistry
PDF Search Title:
Topics in Current ChemistryOriginal File Name Searched:
Elemental-Sulfur-und-Sulfur-Rich-Compounds-I.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |