
PDF Publication Title:
Text from PDF Page: 002
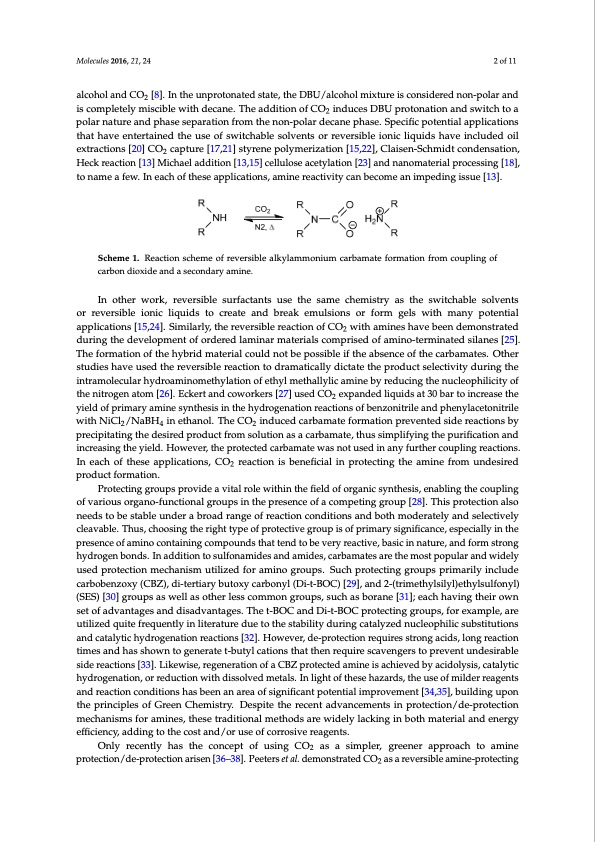
Molecules 2016, 21, 24 2 of 11 Molecules 2016, 21, 24 2 of 10 alcohol and CO2 [8]. In the unprotonated state, the DBU/alcohol mixture is considered non-polar and iscompletelymisciblewithdecane.TheadditionofCO inducesDBUprotonationandswitchtoa is completely miscible with decane. The addition of CO2 induces DBU protonation and switch to a polar nature and phase separation from the non-polar decane phase. Specific potential applications polar nature and phase separation from the non-polar decane phase. Specific potential applications that have entertained the use of switchable solvents or reversible ionic liquids have included oil that have entertained the use of switchable solvents or reversible ionic liquids have included oil extractions [20] CO capture [17,21] styrene polymerization [15,22], Claisen-Schmidt condensation, extractions [20] CO2 capture [17,21] styrene polymerization [15,22], Claisen-Schmidt condensation, Heck reaction [13] Michael addition [13,15] cellulose acetylation [23] and nanomaterial processing [18], Heck reaction [13] Michael addition [13,15] cellulose acetylation [23] and nanomaterial processing [18], to name a few. In each of these applications, amine reactivity can become an impeding issue [13]. to name a few. In each of these applications, amine reactivity can become an impeding issue [13]. Scheme 1. Reaction scheme of reversible alkylammonium carbamate formation from coupling of Scheme 1. Reaction scheme of reversible alkylammonium carbamate formation from coupling of carbon dioxide and a secondary amine. carbon dioxide and a secondary amine. In other work, reversible surfactants use the same chemistry as the switchable solvents or reversible In other work, reversible surfactants use the same chemistry as the switchable solvents ionic liquids to create and break emulsions or form gels with many potential applications [15,24]. or reversible ionic liquids to create and break emulsions or form gels with many potential Similarly, the reversible reaction of CO2 with amines have been demonstrated during the development applications [15,24]. Similarly, the reversible reaction of CO2 with amines have been demonstrated of ordered laminar materials comprised of amino-terminated silanes [25]. The formation of the hybrid during the development of ordered laminar materials comprised of amino-terminated silanes [25]. material could not be possible if the absence of the carbamates. Other studies have used the reversible The formation of the hybrid material could not be possible if the absence of the carbamates. Other reaction to dramatically dictate the product selectivity during the intramolecular hydroaminomethylation studies have used the reversible reaction to dramatically dictate the product selectivity during the of ethyl methallylic amine by reducing the nucleophilicity of the nitrogen atom [26]. Eckert and intramolecular hydroaminomethylation of ethyl methallylic amine by reducing the nucleophilicity of coworkers [27] used CO2 expanded liquids at 30 bar to increase the yield of primary amine synthesis the nitrogen atom [26]. Eckert and coworkers [27] used CO2 expanded liquids at 30 bar to increase the in the hydrogenation reactions of benzonitrile and phenylacetonitrile with NiCl2/NaBH4 in ethanol. yield of primary amine synthesis in the hydrogenation reactions of benzonitrile and phenylacetonitrile The CO2 induced carbamate formation prevented side reactions by precipitating the desired product with NiCl2/NaBH4 in ethanol. The CO2 induced carbamate formation prevented side reactions by from solution as a carbamate, thus simplifying the purification and increasing the yield. However, precipitating the desired product from solution as a carbamate, thus simplifying the purification and the protected carbamate was not used in any further coupling reactions. In each of these applications, increasing the yield. However, the protected carbamate was not used in any further coupling reactions. CO2 reaction is beneficial in protecting the amine from undesired product formation. In each of these applications, CO2 reaction is beneficial in protecting the amine from undesired Protecting groups provide a vital role within the field of organic synthesis, enabling the coupling product formation. of various organo-functional groups in the presence of a competing group [28]. This protection also Protecting groups provide a vital role within the field of organic synthesis, enabling the coupling needs to be stable under a broad range of reaction conditions and both moderately and selectively of various organo-functional groups in the presence of a competing group [28]. This protection also cleavable. Thus, choosing the right type of protective group is of primary significance, especially in needs to be stable under a broad range of reaction conditions and both moderately and selectively the presence of amino containing compounds that tend to be very reactive, basic in nature, and form cleavable. Thus, choosing the right type of protective group is of primary significance, especially in the strong hydrogen bonds. In addition to sulfonamides and amides, carbamates are the most popular presence of amino containing compounds that tend to be very reactive, basic in nature, and form strong and widely used protection mechanism utilized for amino groups. Such protecting groups primarily include hydrogen bonds. In addition to sulfonamides and amides, carbamates are the most popular and widely carbobenzoxy (CBZ), di-tertiary butoxy carbonyl (Di-t-BOC) [29], and 2-(trimethylsilyl)ethylsulfonyl) used protection mechanism utilized for amino groups. Such protecting groups primarily include (SES) [30] groups as well as other less common groups, such as borane [31]; each having their own carbobenzoxy (CBZ), di-tertiary butoxy carbonyl (Di-t-BOC) [29], and 2-(trimethylsilyl)ethylsulfonyl) set of advantages and disadvantages. The t-BOC and Di-t-BOC protecting groups, for example, are (SES) [30] groups as well as other less common groups, such as borane [31]; each having their own utilized quite frequently in literature due to the stability during catalyzed nucleophilic substitutions set of advantages and disadvantages. The t-BOC and Di-t-BOC protecting groups, for example, are and catalytic hydrogenation reactions [32]. However, de-protection requires strong acids, long utilized quite frequently in literature due to the stability during catalyzed nucleophilic substitutions reaction times and has shown to generate t-butyl cations that then require scavengers to prevent and catalytic hydrogenation reactions [32]. However, de-protection requires strong acids, long reaction undesirable side reactions [33]. Likewise, regeneration of a CBZ protected amine is achieved by times and has shown to generate t-butyl cations that then require scavengers to prevent undesirable acidolysis, catalytic hydrogenation, or reduction with dissolved metals. In light of these hazards, the side reactions [33]. Likewise, regeneration of a CBZ protected amine is achieved by acidolysis, catalytic use of milder reagents and reaction conditions has been an area of significant potential improvement hydrogenation, or reduction with dissolved metals. In light of these hazards, the use of milder reagents [34,35], building upon the principles of Green Chemistry. Despite the recent advancements in and reaction conditions has been an area of significant potential improvement [34,35], building upon protection/de-protection mechanisms for amines, these traditional methods are widely lacking in both the principles of Green Chemistry. Despite the recent advancements in protection/de-protection material and energy efficiency, adding to the cost and/or use of corrosive reagents. mechanisms for amines, these traditional methods are widely lacking in both material and energy Only recently has the concept of using CO2 as a simpler, greener approach to amine protection/ efficiency, adding to the cost and/or use of corrosive reagents. de-protection arisen [36–38]. Peeters et al. demonstrated CO2 as a reversible amine-protecting agent Only recently has the concept of using CO2 as a simpler, greener approach to amine in selective Michael additions and acylations [36]. Specifically, the reaction of primary amines was protection/de-protection arisen [36–38]. Peeters et al. demonstrated CO2 as a reversible amine-protecting inhibited in favor of normally less reactive sulfonamides, cyclic secondary amines, or β-ketoesters for the Michael addition and complete inhibition of benzylamine acylation was achieved, effectively favoring alcohol conversion without significant byproduct. Ethier et al. investigated the solvent effect and addition of other bases in addition to DBU on the protection of benzylamines with CO2 [37]. In eachPDF Image | Amines vs Nucleophilic Substitution Reversible Reaction CO2
PDF Search Title:
Amines vs Nucleophilic Substitution Reversible Reaction CO2Original File Name Searched:
molecules-21-00024.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |