
PDF Publication Title:
Text from PDF Page: 004
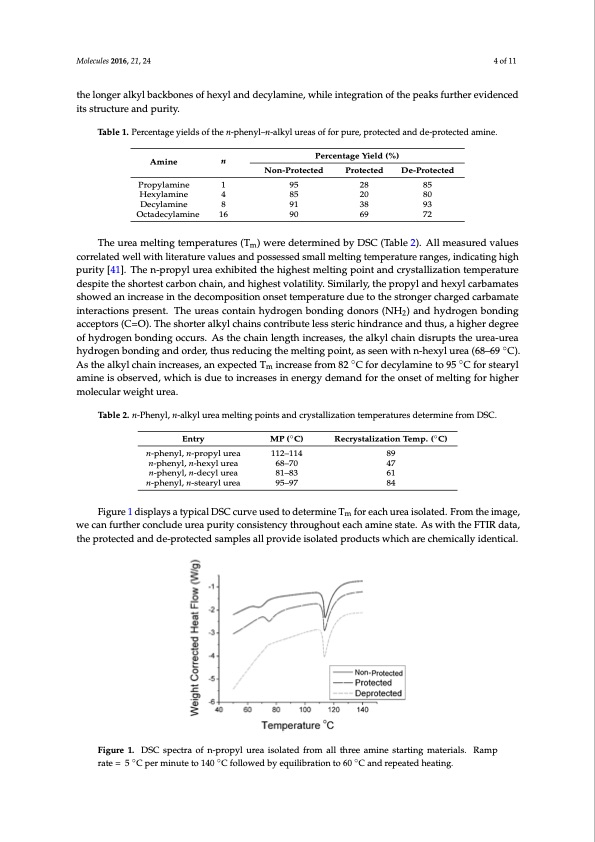
Molecules 2016, 21, 24 4 of 11 the longer alkyl backbones of hexyl and decylamine, while integration of the peaks further evidenced its structure and purity. Table 1. Percentage yields of the n-phenyl–n-alkyl ureas of for pure, protected and de-protected amine. Amine n Percentage Yield (%) Non-Protected Protected De-Protected Molecules 2016, 21, 24 Hexylamine Propylamine 1 95 4 85 8 91 28 85 20 80 38 93 4 of 10 differences in spectra were the chemical shifts at 1.3 ppm attributed to the longer alkyl backbones of Decylamine Octadecylamine 16 90 69 72 hexyl and decylamine, while integration of the peaks further evidenced its structure and purity. The urea melting temperatures (Tm) were determined by DSC (Table 2). All measured values The urea melting temperatures (Tm) were determined by DSC (Table 2). All measured values correlated well with literature values and possessed small melting temperature ranges, indicating correlated well with literature values and possessed small melting temperature ranges, indicating high high purity [41]. The n-propyl urea exhibited the highest melting point and crystallization temperature purity [41]. The n-propyl urea exhibited the highest melting point and crystallization temperature despite the shortest carbon chain, and highest volatility. Similarly, the propyl and hexyl carbamates despite the shortest carbon chain, and highest volatility. Similarly, the propyl and hexyl carbamates showed an increase in the decomposition onset temperature due to the stronger charged carbamate showed an increase in the decomposition onset temperature due to the stronger charged carbamate interactions present. The ureas contain hydrogen bonding donors (NH2) and hydrogen bonding interactions present. The ureas contain hydrogen bonding donors (NH2) and hydrogen bonding acceptors (C=O). The shorter alkyl chains contribute less steric hindrance and thus, a higher degree acceptors (C=O). The shorter alkyl chains contribute less steric hindrance and thus, a higher degree of hydrogen bonding occurs. As the chain length increases, the alkyl chain disrupts the urea-urea of hydrogen bonding occurs. As the chain length increases, the alkyl chain disrupts the urea-urea hydrogen bonding and order, thus reducing the melting point, as seen with n-hexyl urea (68–69 °C). ̋As hydrogen bonding and order, thus reducing the melting point, as seen with n-hexyl urea (68–69 C). the alkyl chain increases, an expected Tm increase from 82 °C for ̋ decylamine to 95 °C for ̋stearyl amine As the alkyl chain increases, an expected Tm increase from 82 C for decylamine to 95 C for stearyl is observed, which is due to increases in energy demand for the onset of melting for higher molecular amine is observed, which is due to increases in energy demand for the onset of melting for higher weight urea. molecular weight urea. Table 2. n-Phenyl, n-alkyl urea melting points and crystallization temperatures determine from DSC. Table 2. n-Phenyl, n-alkyl urea melting points and crystallization temperatures determine from DSC. Entry MP (°C) Recrystalization Temp. (°C) Entry MP ( ̋C) 112–114 Recrystalization Temp. ( ̋C) n-phenyl, n-propyl urea n-phenyl, n-propyl urea n-phenyl, n-hexyl urea n-phenyl, n-hexyl urea n-phenyl, n-decyl urea n-phenyl, n-decyl urea n-phenyl, n-stearyl urea n-phenyl, n-stearyl urea 112–114 89 47 61 84 89 47 61 84 68–70 68–70 81–83 81–83 95–97 95–97 for each urea isolated. From the image, we can further conclude urea purity consistency throughout each amine state. As with the FTIR data, Figure 1 displays a typical DSC curve used to determine T Figure 1 displays a typical DSC curve used to determine Tm for each urea isolated. From the image, we can further conclude urea purity consistency throughout each amine state. As with the FTIR data, the protected and de-protected samples all provide isolated products which are chemically identical. the protected and de-protected samples all provide isolated products which are chemically identical. Figure 1. DSC spectra of n-propyl urea isolated from all three amine starting materials. Ramp rate = Figure 1. DSC spectra of n-propyl urea isolated from all three amine starting materials. Ramp 5 °C per ̋minute to 140 °C follo ̋wed by equilibration to 60 °C and ̋repeated heating. rate = 5 C per minute to 140 C followed by equilibration to 60 C and repeated heating. 2.2. Synthesis of n-Propyl, Benzophenone(BP) Imine Titanium(IV) isopropoxide is a low cost mediator in BP imine synthesis [42–46]. According to Figure 2, the initial BP concentration of 0.45 M is slowly reduced to 0.26 M after 3 h and finally 0.11 M after 24 h as the reaction proceeds. Gas chromatography (GC) was used to determine an overall yield of 75% for the non-protected amine while a 25% yield is obtained for the protected and 50% forPDF Image | Amines vs Nucleophilic Substitution Reversible Reaction CO2
PDF Search Title:
Amines vs Nucleophilic Substitution Reversible Reaction CO2Original File Name Searched:
molecules-21-00024.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |