
PDF Publication Title:
Text from PDF Page: 005
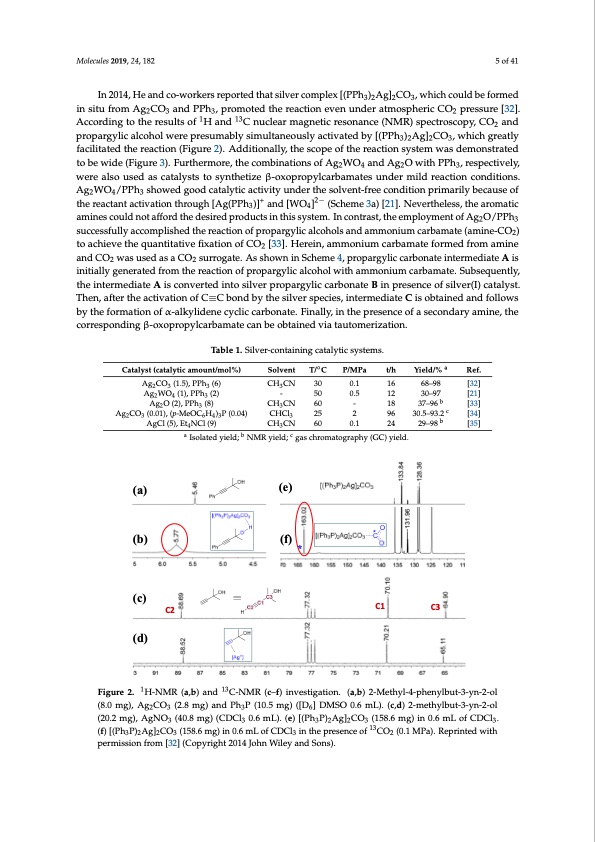
Molecules 2019, 24, x FOR PEER REVIEW 5 of 42 Molecules 2019, 24, 182 5 of 41 In 2014, He and co-workers reported that silver complex [(PPh3)2Ag]2CO3, which could be formed in situ from Ag2CO3 and PPh3, promoted the reaction even under atmospheric CO2 pressure [32]. According to the results of 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, CO2 In 2014, He and co-workers reported that silver complex [(PPh3)2Ag]2CO3, which could be formed and propargylic alcohol were presumably simultaneously activated by [(PPh3)2Ag]2CO3, which in situ from Ag2CO3 and PPh3, promoted the reaction even under atmospheric CO2 pressure [32]. greatly facilitated the reac1tion (Fi1g3ure 2). Additionally, the scope of the reaction system was According to the results of H and C nuclear magnetic resonance (NMR) spectroscopy, CO2 and demonstrated to be wide (Figure 3). Furthermore, the combinations of Ag2WO4 and Ag2O with PPh3, propargylic alcohol were presumably simultaneously activated by [(PPh3)2Ag]2CO3, which greatly respectively, were also used as catalysts to synthetize β-oxopropylcarbamates under mild reaction facilitated the reaction (Figure 2). Additionally, the scope of the reaction system was demonstrated conditions. Ag2WO4/PPh3 showed good catalytic activity under the solvent-free condition primarily to be wide (Figure 3). Furthermore, the combinations of Ag2WO4 and Ag2O with PPh3, respectively, because of the reactant activation through [Ag(PPh3)]+ and [WO4]2− (Scheme 3a) [21]. Nevertheless, were also used as catalysts to synthetize β-oxopropylcarbamates under mild reaction conditions. the aromatic amines could not afford the desired products in this system. In contrast, the Ag2WO4/PPh3 showed good catalytic activity under the solvent-free condition primarily because of employment of Ag2O/PPh3 successfully+accomplish2e−d the reaction of propargylic alcohols and the reactant activation through [Ag(PPh3)] and [WO4] (Scheme 3a) [21]. Nevertheless, the aromatic ammonium carbamate (amine-CO2) to achieve the quantitative fixation of CO2 [33]. Herein, amines could not afford the desired products in this system. In contrast, the employment of Ag2O/PPh3 ammonium carbamate formed from amine and CO2 was used as a CO2 surrogate. As shown in successfully accomplished the reaction of propargylic alcohols and ammonium carbamate (amine-CO2) Scheme 4, propargylic carbonate intermediate A is initially generated from the reaction of to achieve the quantitative fixation of CO2 [33]. Herein, ammonium carbamate formed from amine propargylic alcohol with ammonium carbamate. Subsequently, the intermediate A is converted into and CO2 was used as a CO2 surrogate. As shown in Scheme 4, propargylic carbonate intermediate A is silver propargylic carbonate B in presence of silver(I) catalyst. Then, after the activation of C≡C bond initially generated from the reaction of propargylic alcohol with ammonium carbamate. Subsequently, by the silver species, intermediate C is obtained and follows by the formation of α-alkylidene cyclic the intermediate A is converted into silver propargylic carbonate B in presence of silver(I) catalyst. carbonate. Finally, in the presence of a secondary amine, the corresponding β-oxopropylcarbamate Then, after the activation of C≡C bond by the silver species, intermediate C is obtained and follows can be obtained via tautomerization. by the formation of α-alkylidene cyclic carbonate. Finally, in the presence of a secondary amine, the corresponding β-oxopropylcarbamate can be obtained via tautomerization. Table 1. Silver-containing catalytic systems. Catalyst(catalyticamount/mol%)TableS1o.lSveilnvter-contaTi/n°CingcatalyPt/iMcPsyastems.t/h Yield/%a Ref. Ag2CO3 (1.5), PPh3 (6) CH3CN 30 0.1 16 68-98 [32] Ref. [21] [33] Catalyst (catalytic amount/mol%) Solvent T/◦C P/MPa t/h Yield/% a Ag2WO4 (1), PPh3 (2) - 50 0.5 12 30-97 0.1 16 68–98 b - 18 37-96 0.5 12 30–97 Ag2CO3 (1.5), PPh3 (6) CH3CN 30 [32] Ag2O (2), PPh3 (8) CH3CN 60 Ag2WO4 (1), PPh3 (2) Ag2CO3 (0.01), (p-MeOC6H4)3P Ag O (2), PPh (8) - 50 CH CN 60 - 18 37–96 b [21] c [33] [34] (0.04) c bb 2 3 CHCl3 325 2 96 30.5-93.2 [34] [35] [35] Ag2CO3 (0.01), (p-MeOC6H4)3P (0.04) AgCl (5)A, EgtC4Nl (C5)l,(E9)t4NCl (9) CH3CN CHCl3 25 CH3CN60 60 2 96 0.10.1 2244 30.5–93.2 29–298-98 aa bb c IsoIlsaotleadtedyiyeiledld;;NNMRRyiield; gaschrroomataotgorgarpahpyh(yGC(G)Cyi)elydi.eld. (a) (b) (c) C2 (d) (e) (f) * C1 C3 1 13 Figure 2. 1 H-NMR (a,b) and13 C-NMR (c–f) investigation. (a,b) 2-Methyl-4-phenylbut-3-yn-2-ol Figure 2. H-NMR (a, b) and C-NMR (c–f) investigation. (a,b) 2-Methyl-4-phenylbut-3-yn-2-ol (8.0 (8.0 mg), Ag2CO3 (2.8 mg) and Ph3P (10.5 mg) ([D6] DMSO 0.6 mL). (c,d) 2-methylbut-3-yn-2-ol mg), Ag2CO3 (2.8 mg) and Ph3P (10.5 mg) ([D6] DMSO 0.6 mL). (c,d) 2-methylbut-3-yn-2-ol (20.2 mg), (20.2 mg), AgNO3 (40.8 mg) (CDCl3 0.6 mL). (e) [(Ph3P)2Ag]2CO3 (158.6 mg) in 0.6 mL of CDCl3. AgNO3 (40.8 mg) (CDCl3 0.6 mL). (e) [(Ph3P)2Ag]2CO3 (158.6 mg) in 0.6 mL of CDCl3. (f) (f) [(Ph P) Ag] CO (158.6 mg) in 0.6 mL of CDCl in the presence of 13CO (0.1 MPa). Reprinted with 3223 3 132 [(Ph3P)2Ag]2CO3 (158.6 mg) in 0.6 mL of CDCl3 in the presence of CO2 (0.1 MPa). Reprinted with permission from [32] (Copyright 2014 John Wiley and Sons). permission from [32] (Copyright 2014 John Wiley and Sons).PDF Image | Catalytic Conversion of Carbon Dioxide through C-N Bond
PDF Search Title:
Catalytic Conversion of Carbon Dioxide through C-N BondOriginal File Name Searched:
molecules-24-00182.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |