
PDF Publication Title:
Text from PDF Page: 119
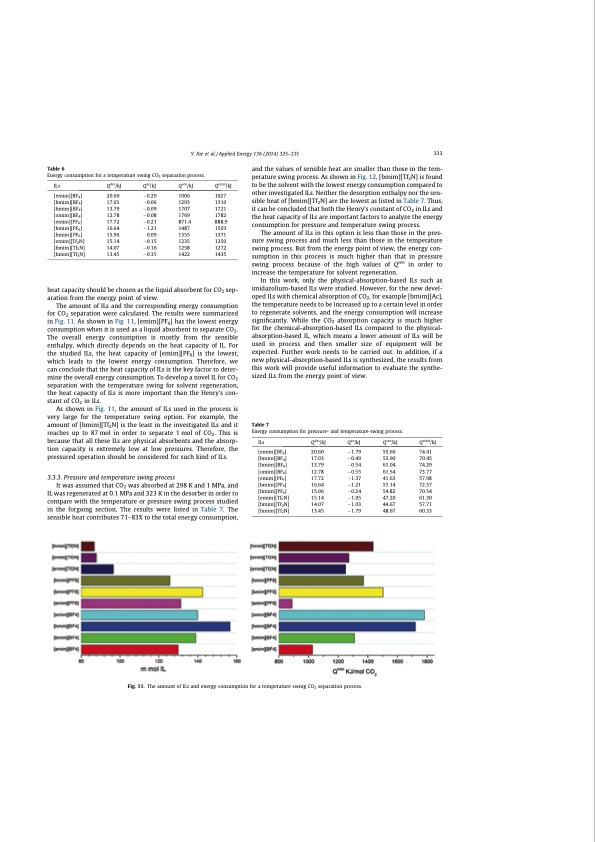
Table 6 Energy consumption for a temperature swing CO2 separation process. and the values of sensible heat are smaller than those in the tem- perature swing process. As shown in Fig. 12, [bmim][Tf2N] is found to be the solvent with the lowest energy consumption compared to other investigated ILs. Neither the desorption enthalpy nor the sen- sible heat of [bmim][Tf2N] are the lowest as listed in Table 7. Thus, it can be concluded that both the Henry’s constant of CO2 in ILs and the heat capacity of ILs are important factors to analyze the energy consumption for pressure and temperature swing process. The amount of ILs in this option is less than those in the pres- sure swing process and much less than those in the temperature swing process. But from the energy point of view, the energy con- sumption in this process is much higher than that in pressure swing process because of the high values of Qsen in order to increase the temperature for solvent regeneration. In this work, only the physical-absorption-based ILs such as imidazolium-based ILs were studied. However, for the new devel- oped ILs with chemical absorption of CO2, for example [bmim][Ac], the temperature needs to be increased up to a certain level in order to regenerate solvents, and the energy consumption will increase significantly. While the CO2 absorption capacity is much higher for the chemical-absorption-based ILs compared to the physical- absorption-based IL, which means a lower amount of ILs will be used in process and then smaller size of equipment will be expected. Further work needs to be carried out. In addition, if a new physical-absorption-based ILs is synthesized, the results from this work will provide useful information to evaluate the synthe- sized ILs from the energy point of view. ILs [emim][BF4] [bmim][BF4] [hmim][BF4] [omim][BF4] [emim][PF6] [bmim][PF6] [hmim][PF6] [emim][Tf2N] [bmim][Tf2N] [hmim][Tf2N] Qdes/kJ Qex/kJ Qsen/kJ Qtotal/kJ 15.14 0.15 1235 14.07 0.16 1258 1272 13.45 0.35 1422 1435 Y. Xie et al. / Applied Energy 136 (2014) 325–335 333 20.60 0.29 1006 1027 17.03 0.06 1293 13.79 0.09 1707 12.78 0.08 1769 1782 17.72 0.21 871.4 888.9 16.64 1.21 1487 1503 15.96 0.09 1355 1371 1310 1721 1250 heat capacity should be chosen as the liquid absorbent for CO2 sep- aration from the energy point of view. The amount of ILs and the corresponding energy consumption for CO2 separation were calculated. The results were summarized in Fig. 11. As shown in Fig. 11, [emim][PF6] has the lowest energy consumption when it is used as a liquid absorbent to separate CO2. The overall energy consumption is mostly from the sensible enthalpy, which directly depends on the heat capacity of IL. For the studied ILs, the heat capacity of [emim][PF6] is the lowest, which leads to the lowest energy consumption. Therefore, we can conclude that the heat capacity of ILs is the key factor to deter- mine the overall energy consumption. To develop a novel IL for CO2 separation with the temperature swing for solvent regeneration, the heat capacity of ILs is more important than the Henry’s con- stant of CO2 in ILs. As shown in Fig. 11, the amount of ILs used in the process is very large for the temperature swing option. For example, the amount of [hmim][Tf2N] is the least in the investigated ILs and it reaches up to 87 mol in order to separate 1 mol of CO2. This is because that all these ILs are physical absorbents and the absorp- tion capacity is extremely low at low pressures. Therefore, the pressured operation should be considered for such kind of ILs. 3.3.3. Pressure and temperature swing process It was assumed that CO2 was absorbed at 298 K and 1 MPa, and IL was regenerated at 0.1 MPa and 323 K in the desorber in order to compare with the temperature or pressure swing process studied in the forgoing section. The results were listed in Table 7. The sensible heat contributes 71–83% to the total energy consumption, Table 7 Energy consumption for pressure- and temperature-swing process. ILs [emim][BF4] [bmim][BF4] [hmim][BF4] [omim][BF4] [emim][PF6] [bmim][PF6] [hmim][PF6] [emim][Tf2N] [bmim][Tf2N] [hmim][Tf2N] Qdes/kJ Qex/kJ Qsen/kJ 20.60 1.79 55.60 17.03 0.49 53.90 13.79 0.54 61.04 12.78 0.55 61.54 17.72 1.37 41.63 16.64 1.21 57.14 15.96 0.24 54.82 15.14 1.05 47.20 14.07 1.03 44.67 13.45 1.79 48.67 Qtotal/kJ 74.41 70.45 74.29 73.77 57.98 72.57 70.54 61.30 57.71 60.33 Fig. 11. The amount of ILs and energy consumption for a temperature swing CO2 separation process.PDF Image | CO2 Separation with Ionic Liquids
PDF Search Title:
CO2 Separation with Ionic LiquidsOriginal File Name Searched:
co2-separation-ionic-liquids.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |