
PDF Publication Title:
Text from PDF Page: 127
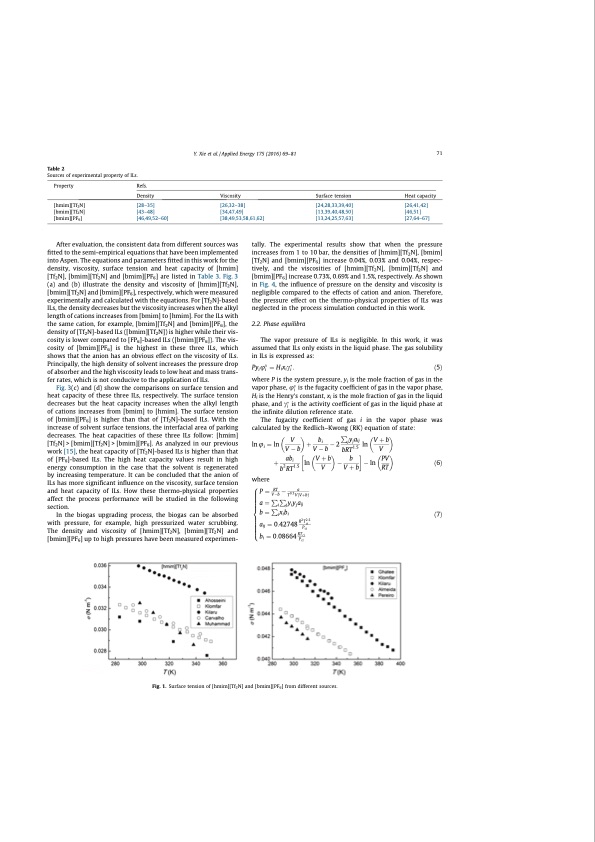
Table 2 Sources of experimental property of ILs. Property [hmim][Tf2N] [bmim][Tf2N] [bmim][PF6] Refs. Density Viscosity Surface tension Heat capacity [28–35] [26,32–38] [24,28,33,39,40] [26,41,42] [43–48] [34,47,49] [13,39,40,48,50] [46,51] [46,49,52–60] [38,49,53,58,61,62] [13,24,25,57,63] [27,64–67] Y. Xie et al. / Applied Energy 175 (2016) 69–81 71 After evaluation, the consistent data from different sources was fitted to the semi-empirical equations that have been implemented into Aspen. The equations and parameters fitted in this work for the density, viscosity, surface tension and heat capacity of [hmim] [Tf2N], [bmim][Tf2N] and [bmim][PF6] are listed in Table 3. Fig. 3 (a) and (b) illustrate the density and viscosity of [hmim][Tf2N], [bmim][Tf2N] and [bmim][PF6], respectively, which were measured experimentally and calculated with the equations. For [Tf2N]-based ILs, the density decreases but the viscosity increases when the alkyl length of cations increases from [bmim] to [hmim]. For the ILs with the same cation, for example, [bmim][Tf2N] and [bmim][PF6], the density of [Tf2N]-based ILs ([bmim][Tf2N]) is higher while their vis- cosity is lower compared to [FP6]-based ILs ([bmim][PF6]). The vis- cosity of [bmim][PF6] is the highest in these three ILs, which shows that the anion has an obvious effect on the viscosity of ILs. Principally, the high density of solvent increases the pressure drop of absorber and the high viscosity leads to low heat and mass trans- fer rates, which is not conducive to the application of ILs. Fig. 3(c) and (d) show the comparisons on surface tension and heat capacity of these three ILs, respectively. The surface tension decreases but the heat capacity increases when the alkyl length of cations increases from [bmim] to [hmim]. The surface tension of [bmim][PF6] is higher than that of [Tf2N]-based ILs. With the increase of solvent surface tensions, the interfacial area of parking decreases. The heat capacities of these three ILs follow: [hmim] [Tf2N] > [bmim][Tf2N] > [bmim][PF6]. As analyzed in our previous work [15], the heat capacity of [Tf2N]-based ILs is higher than that of [PF6]-based ILs. The high heat capacity values result in high energy consumption in the case that the solvent is regenerated by increasing temperature. It can be concluded that the anion of ILs has more significant influence on the viscosity, surface tension and heat capacity of ILs. How these thermo-physical properties affect the process performance will be studied in the following section. In the biogas upgrading process, the biogas can be absorbed with pressure, for example, high pressurized water scrubbing. The density and viscosity of [hmim][Tf2N], [bmim][Tf2N] and [bmim][PF6] up to high pressures have been measured experimen- tally. The experimental results show that when the pressure increases from 1 to 10 bar, the densities of [hmim][Tf2N], [bmim] [Tf2N] and [bmim][PF6] increase 0.04%, 0.03% and 0.04%, respec- tively, and the viscosities of [hmim][Tf2N], [bmim][Tf2N] and [bmim][PF6] increase 0.73%, 0.69% and 1.5%, respectively. As shown in Fig. 4, the influence of pressure on the density and viscosity is negligible compared to the effects of cation and anion. Therefore, the pressure effect on the thermo-physical properties of ILs was neglected in the process simulation conducted in this work. 2.2. Phase equilibra The vapor pressure of ILs is negligible. In this work, it was assumed that ILs only exists in the liquid phase. The gas solubility in ILs is expressed as: Pyiumi 1⁄4 Hixici : ð5Þ where P is the system pressure, yi is the mole fraction of gas in the vapor phase, umi is the fugacity coefficient of gas in the vapor phase, Hi is the Henry’s constant, xi is the mole fraction of gas in the liquid phase, and ci is the activity coefficient of gas in the liquid phase at the infinite dilution reference state. The fugacity coefficient of gas i in the vapor phase was calculated by the Redlich–Kwong (RK) equation of state: P ln u 1⁄4 ln V þ bi 2 jyjaij ln V þ b i where Vb Vb bRT1:5 V þ abi ln Vþb b ln PV ð6Þ b2RT1:5 V Vþb RT 8>P1⁄4RT a > Vb T0:5VðVþbÞ > a 1⁄4 P P yi yj aij >< Pi j >b1⁄4 ixibi > a 1⁄4 0:42748 R2 T 2:5 ð7Þ > ij P ci ci >:bi 1⁄40:08664RTci Pci Fig. 1. Surface tension of [hmim][Tf2N] and [bmim][PF6] from different sources.PDF Image | CO2 Separation with Ionic Liquids
PDF Search Title:
CO2 Separation with Ionic LiquidsOriginal File Name Searched:
co2-separation-ionic-liquids.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |